Patients with high-grade alectinib-induced skin rash: How do we desensitize these patients? A case report and review of literature
- PMID: 33149916
- PMCID: PMC7586266
- DOI: 10.1177/2050313X20966895
Patients with high-grade alectinib-induced skin rash: How do we desensitize these patients? A case report and review of literature
Abstract
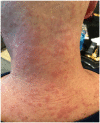
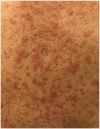
With the advent of targeted therapy for non-small-cell lung cancer, there are many new available treatment options for patients whose cancer harbors an actionable mutation or alteration. These new medications come with numerous side effects, for some of which, the management is not well defined. Alectinib is a second-generation tyrosine kinase inhibitor approved for stage-IV lung adenocarcinoma with anaplastic lymphoma kinase gene rearrangement. Severe (⩾Grade 3) skin rash is a rare side effect of alectinib. Reintroducing alectinib in patients with severe skin rash is not well defined in the medical literature. While other case reports have outlined their approach and desensitization protocol, the maximum dose that patients were titrated up to in a desensitization protocol was 300 mg twice daily. Here, we report a case of Grade 3 skin rash secondary to alectinib, and our experience in managing the rash and reintroducing alectinib with a unique desensitization protocol to a max of 600 mg twice daily (full dose). This case could provide further guidance to oncologists managing patients with this adverse event and may aid in reducing concerns to both patients and physicians about recurrence of skin rash at the maximum dose.
Keywords: Alectinib; anaplastic lymphoma kinase; desensitization; skin rash.
© The Author(s) 2020.
Conflict of interest statement
Declaration of conflicting of interests: R.M. discloses advisory board in AstraZeneca and advisory board for Guardant Health, Novocure and Takeda and consultancy in AstraZeneca. Y.L. discloses advisory board in AstraZeneca and Novocure; consultancy in AstraZeneca; honoraria in Clarion Health Care; research funding support from Merck, MacroGenics, Tolero Pharmaceuticals, AstraZeneca, Vaccinex, Blueprint Medicines and Sun Pharma; and advanced research from Bristol-Myers Squibb, Kyowa Pharmaceuticals and Tesaro.
Figures
References
-
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 2017; 377: 829–838. - PubMed
-
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014; 371: 2167–2177. - PubMed
-
- Ou SI, Gadgeel SM, Barlesi F, et al. Pooled overall survival and safety data from the pivotal phase II studies (NP28673 and NP28761) of alectinib in ALK-positive non-small-cell lung cancer. Lung Cancer 2020; 139: 22–27. - PubMed
Publication types
LinkOut - more resources
Full Text Sources