Analysis of retinal nerve fiber layer birefringence in patients with glaucoma and diabetic retinopathy by polarization sensitive OCT
- PMID: 33149966
- PMCID: PMC7587266
- DOI: 10.1364/BOE.402475
Analysis of retinal nerve fiber layer birefringence in patients with glaucoma and diabetic retinopathy by polarization sensitive OCT
Abstract
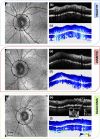
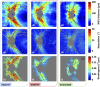
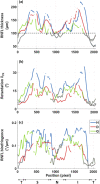
The retinal nerve fiber layer (RNFL) is a fibrous tissue that shows form birefringence. This optical tissue property is related to the microstructure of the nerve fiber axons that carry electrical signals from the retina to the brain. Ocular diseases that are known to cause neurologic changes, like glaucoma or diabetic retinopathy (DR), might alter the birefringence of the RNFL, which could be used for diagnostic purposes. In this pilot study, we used a state-of-the-art polarization sensitive optical coherence tomography (PS-OCT) system with an integrated retinal tracker to analyze the RNFL birefringence in patients with glaucoma, DR, and in age-matched healthy controls. We recorded 3D PS-OCT raster scans of the optic nerve head area and high-quality averaged circumpapillary PS-OCT scans, from which RNFL thickness, retardation and birefringence were derived. The precision of birefringence measurements was 0.005°/µm. As compared to healthy controls, glaucoma patients showed a slightly reduced birefringence (0.129 vs. 0.135°/µm), although not statistically significant. The DR patients, however, showed a stronger reduction of RNFL birefringence (0.103 vs. 0.135°/µm) which was highly significant. This result might open new avenues into early diagnosis of DR and related neurologic changes.
Published by The Optical Society under the terms of the Creative Commons Attribution 4.0 License. Further distribution of this work must maintain attribution to the author(s) and the published article’s title, journal citation, and DOI.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Sabanayagam C., Banu R., Chee M. L., Lee R., Wang Y. X., Tan G., Jonas J. B., Lamoureux E. L., Cheng C.-Y., Klein B. E. K., Mitchell P., Klein R., Cheung C. M. G., Wong T. Y., “Incidence and progression of diabetic retinopathy: a systematic review,” Lancet Diabetes Endocrinol. 7(2), 140–149 (2019). 10.1016/S2213-8587(18)30128-1 - DOI - PubMed
-
- Corcóstegui B., Durán S., González-Albarrán M. O., Hernández C., Ruiz-Moreno J. M., Salvador J., Udaondo P., Simó R., “Update on Diagnosis and Treatment of Diabetic Retinopathy: A Consensus Guideline of the Working Group of Ocular Health (Spanish Society of Diabetes and Spanish Vitreous and Retina Society),” J. Ophthalmol. 2017, 1–10 (2017). 10.1155/2017/8234186 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
