Depth-resolved Mueller matrix polarimetry microscopy of the rat cornea
- PMID: 33150000
- PMCID: PMC7587284
- DOI: 10.1364/BOE.402201
Depth-resolved Mueller matrix polarimetry microscopy of the rat cornea
Abstract
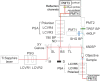
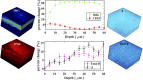
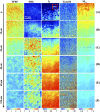
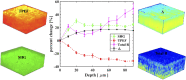
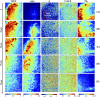
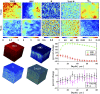
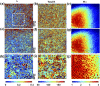
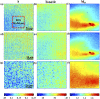
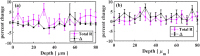
Mueller matrix polarimetry (MMP) is a promising linear imaging modality that can enable visualization and measurement of the polarization properties of the cornea. Although the distribution of corneal birefringence has been reported, depth resolved MMP imaging of the cornea has not been archived and remains challenging. In this work, we perform depth-resolved imaging of the cornea using an improved system that combines Mueller matrix reflectance and transmission microscopy together with nonlinear microscopy utilizing second harmonic generation (SHG) and two photon excitation fluorescence (TPEF). We show that TPEF can reveal corneal epithelial cellular network while SHG can highlight the presence of corneal stromal lamellae. We then demonstrate that, in confocal reflectance measurement, as depth increases from 0 to 80 μm both corneal depolarization and retardation increase. Furthermore, it is shown that the spatial distribution of corneal depolarization and retardation displays similar complexity in both reflectance (confocal and non-confocal) and transmission measurement, likely due to the strong degree of heterogeneity in the stromal lamellae.
© 2020 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources
