Safety and immunogenicity of Vi-DT conjugate vaccine among 6-23-month-old children: Phase II, randomized, dose-scheduling, observer-blind Study
- PMID: 33150320
- PMCID: PMC7599314
- DOI: 10.1016/j.eclinm.2020.100540
Safety and immunogenicity of Vi-DT conjugate vaccine among 6-23-month-old children: Phase II, randomized, dose-scheduling, observer-blind Study
Abstract
Background: Typhoid causes significant mortality among young children in resource-limited settings. Conjugate typhoid vaccines could significantly reduce typhoid-related child deaths, but only one WHO-prequalified typhoid conjugate vaccine exists for young children. To address this gap, we investigated the safety, immunogenicity and dose-scheduling of Vi-DT typhoid conjugate vaccine among children aged 6-23 months.
Methods: In this single center, observer blind, phase II trial, participants were randomly assigned (2:2:1) to receive one or two doses of Vi-DT or comparator vaccine. Anti-Vi IgG titer and geometric mean titers (GMT) were determined at 0, 4, 24 and 28 weeks. Data were analyzed using per-protocol and immunogenicity (a subset of intention-to-treat analysis) sets. The trial is registered with ClinicalTrials.gov (NCT03527355).
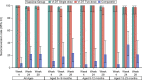
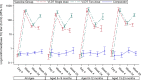
Findings: Between April and July 2018, 285 children were randomized; 114 received one or two doses of Vi-DT while 57 received comparator. 277 completed the study follow-up per protocol; 112 and 110 from single- and two-dose Vi-DT schedules, respectively and 55 from the placebo group were included in the per protocol analysis. Safety profile is satisfactory. Thirteen serious adverse events were reported during the 28-week follow-up, none of which were related to Vi-DT. The seroconversion rate four weeks after the first dose was 100% (95% CI 98·3-100) in Vi-DT recipients and 7·0% (95% CI 2·8-16·7) in comparator recipients (p<0·0001). Similarly, the seroconversion rate 4 weeks after the second dose was 98·2% (95% CI 93· 6-99·5) and 21·8% (95% CI 13·0-34·4) among Vi-DT and comparator groups, respectively (p<0·0001). Anti-Vi IgG GMT was significantly higher in Vi-DT than in control group at all post-vaccination visits (p<0·0001).
Interpretation: Both single and two doses of Vi-DT vaccine are safe, well tolerated, and immunogenic for infants and toddlers in a moderately endemic setting.
Keywords: Infants and toddlers; Typhoid; Vi-DT; typhoid conjugate vaccine.
© 2020 The Author(s).
Conflict of interest statement
Authors, Dr. Capeding reports grants from International Vaccine Institute, during the conduct of the study., Seon-Young Yang, Ji Hwa Ryu, Inho Cheong, Kyu-Young Shim, Yoonyeong Lee, and Hun Kim are employees of SK BioScience. All other authors declare no conflict to interest.
Figures

Similar articles
-
Safety and immunogenicity of the Vi-DT typhoid conjugate vaccine in healthy volunteers in Nepal: an observer-blind, active-controlled, randomised, non-inferiority, phase 3 trial.Lancet Infect Dis. 2022 Apr;22(4):529-540. doi: 10.1016/S1473-3099(21)00455-2. Epub 2021 Dec 20. Lancet Infect Dis. 2022. PMID: 34942090 Free PMC article. Clinical Trial.
-
Immunogenicity, safety and reactogenicity of a Phase II trial of Vi-DT typhoid conjugate vaccine in healthy Filipino infants and toddlers: A preliminary report.Vaccine. 2020 Jun 9;38(28):4476-4483. doi: 10.1016/j.vaccine.2019.09.074. Epub 2019 Oct 1. Vaccine. 2020. PMID: 31585725 Free PMC article. Clinical Trial.
-
Six-month follow up of a randomized clinical trial-phase I study in Indonesian adults and children: Safety and immunogenicity of Salmonella typhi polysaccharide-diphtheria toxoid (Vi-DT) conjugate vaccine.PLoS One. 2019 Feb 13;14(2):e0211784. doi: 10.1371/journal.pone.0211784. eCollection 2019. PLoS One. 2019. PMID: 30759132 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of Vi-diphtheria toxoid typhoid conjugate vaccine among children below 2 years: a systematic review and meta-analysis.Front Microbiol. 2024 Apr 5;15:1385834. doi: 10.3389/fmicb.2024.1385834. eCollection 2024. Front Microbiol. 2024. PMID: 38646637 Free PMC article.
-
Development of Vi conjugate - a new generation of typhoid vaccine.Expert Rev Vaccines. 2013 Nov;12(11):1273-86. doi: 10.1586/14760584.2013.845529. Expert Rev Vaccines. 2013. PMID: 24156285 Review.
Cited by
-
Prospects of Future Typhoid and Paratyphoid Vaccines in Endemic Countries.J Infect Dis. 2021 Dec 20;224(12 Suppl 2):S770-S774. doi: 10.1093/infdis/jiab393. J Infect Dis. 2021. PMID: 34374785 Free PMC article.
-
Observational analysis of the immunogenicity and safety of various types of spinal muscular atrophy vaccines.Inflammopharmacology. 2024 Apr;32(2):1025-1038. doi: 10.1007/s10787-023-01395-7. Epub 2024 Feb 3. Inflammopharmacology. 2024. PMID: 38308795 Clinical Trial.
-
Safety and immunogenicity of conjugate vaccine for typhoid (Vi-DT): Finding from an observer-blind, active-controlled, randomized, non-inferiority, phase III clinical trial among healthy volunteers.Hum Vaccin Immunother. 2024 Dec 31;20(1):2301631. doi: 10.1080/21645515.2023.2301631. Epub 2024 Jan 8. Hum Vaccin Immunother. 2024. PMID: 38189360 Free PMC article. Clinical Trial.
-
A randomized, observer-blind, controlled phase III clinical trial assessing safety and immunological non-inferiority of Vi-diphtheria toxoid versus Vi-tetanus toxoid typhoid conjugate vaccine in healthy volunteers in eastern Nepal.Hum Vaccin Immunother. 2023 Dec 31;19(1):2203634. doi: 10.1080/21645515.2023.2203634. Hum Vaccin Immunother. 2023. PMID: 37128723 Free PMC article. Clinical Trial.
-
Typhoid & paratyphoid vaccine development in the laboratory: a review & in-country experience.Indian J Med Res. 2024 Sep&Oct;160(3&4):379-390. doi: 10.25259/IJMR_1382_2024. Indian J Med Res. 2024. PMID: 39632634 Free PMC article. Review.
References
-
- GOVPH. Department of Health. 2018Typhoid Morbidity Week 52. Available at:https://www.doh.gov.ph/sites/default/files/statistics/2018_TYPHOID_MW52_... (Accessed in September 2019).
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

