Characterizing benefit from temozolomide in MGMT promoter unmethylated and methylated glioblastoma: a systematic review and meta-analysis
- PMID: 33150334
- PMCID: PMC7596890
- DOI: 10.1093/noajnl/vdaa082
Characterizing benefit from temozolomide in MGMT promoter unmethylated and methylated glioblastoma: a systematic review and meta-analysis
Erratum in
-
Erratum to: Characterizing Benefit from Temozolomide in MGMT Promoter Unmethylated and Methylated Glioblastoma: A Systematic Review and Meta-analysis.Neurooncol Adv. 2021 Jul 10;3(1):vdab095. doi: 10.1093/noajnl/vdab095. eCollection 2021 Jan-Dec. Neurooncol Adv. 2021. PMID: 34258581 Free PMC article.
Abstract
Background: The current standard of care for the management of patients with newly diagnosed glioblastoma (GBM) includes maximal safe resection followed by radiotherapy (RT) with concurrent and adjuvant temozolomide (TMZ). While it is well established that TMZ has better efficacy in patients with MGMT promoter methylation, it remains an area of debate whether TMZ should be omitted when treating GBM patients with unmethylated MGMT.
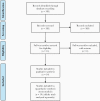
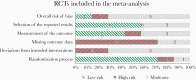
Methods: We conducted a systematic review and meta-analysis to provide separate estimates of median overall survival (OS) and progression-free survival (PFS) for patients with methylated and unmethylated GBM treated with RT with or without TMZ. We searched multiple databases from inception to January 13, 2020.
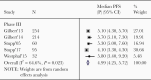
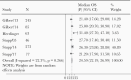
Results: The median OS for patients with unmethylated GBM treated with RT/TMZ pooled from 5 phase III studies (N = 655) was 14.11 months (95% confidence interval [CI], 13.18-15.04) with a median PFS of 4.99 months (95% CI, 4.25-5.72). In contrast, the median OS for patients with methylated GBM pooled from 6 studies (N = 753) was 24.59 months (95% CI, 22.19-26.99) with a median PFS pooled from 7 studies (N = 805) of 9.51 months (95% CI, 7.41-11.61). There is a paucity of prospective data pertaining to OS/PFS in unmethylated patients treated with RT only and therefore a direct comparison was not possible.
Conclusions: This meta-analysis provides estimates of survival for patients with MGMT methylated or unmethylated GBM treated with RT/TMZ. Further research is needed to delineate whether TMZ should be withheld for patients with unmethylated GBM outside of the setting of clinical trials.
Keywords: MGMT; glioblastoma; meta-analysis; systematic review; temozolomide.
© The Author(s) 2020. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Figures


References
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. - PubMed
-
- Taylor JW, Schiff D. Treatment considerations for MGMT-unmethylated glioblastoma. Curr Neurol Neurosci Rep. 2015;15(1):507. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
