EGFR/ErbB2-Targeting Lapatinib Therapy for Aggressive Prolactinomas
- PMID: 33150390
- PMCID: PMC7823257
- DOI: 10.1210/clinem/dgaa805
EGFR/ErbB2-Targeting Lapatinib Therapy for Aggressive Prolactinomas
Abstract
Context: Approximately 10% to 20% of prolactinomas are resistant to dopamine agonist therapy. The ErbB signaling pathway may drive aggressive prolactinoma behavior.
Objective: We evaluated lapatinib, an ErbB1-epidermal growth factor receptor (EGFR)/ErbB2 or human EGFR2 (HER2) tyrosine kinase inhibitor (TKI), in aggressive prolactinomas.
Design: A prospective, phase 2a multicenter trial was conducted.
Setting: This study took place at a tertiary referral pituitary center.
Patients: Study participants included adults with aggressive prolactinomas showing continued tumor growth despite maximally tolerated dopamine agonist therapy.
Intervention: Intervention included oral lapatinib 1250 mg/day for 6 months.
Main outcome measures: The primary end point was 40% reduction in any tumor dimension assessed by magnetic resonance imaging at study end; tumor response was assessed by Response Evaluation Criteria in Solid Tumors criteria. Secondary end points included prolactin (PRL) reduction, correlation of response with EGFR/HER2 expression, and safety.
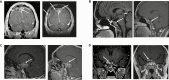
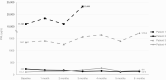
Results: Owing to rigorous inclusion criteria, of 24 planned participants, only 7 consented and 4 were treated. None achieved the primary end point but 3 showed stable disease, including 2 with a 6% increase and 1 with a 16.8% decrease in tumor diameter. PRL response was not always concordant with tumor response, as 2 showed 28% and 59% increases in PRL. The fourth participant had a PRL-secreting carcinoma and withdrew after 3 months of lapatinib because of imaging and PRL progression. EGFR/HER2 expression did not correlate with treatment response. Lapatinib was well tolerated overall, with reversible grade 1 transaminitis in 2 patients, grade 2 rash in 2 patients, and grade 1 asymptomatic bradycardia in 2 patients.
Conclusions: An oral TKI such as lapatinib may be an effective option for a difficult-to-treat patient with an aggressive prolactinoma.
Trial registration: ClinicalTrials.gov NCT00939523.
Keywords: ErbB; HER2; epidermal growth factor receptor; lapatinib; prolactinoma; tyrosine kinase inhibitor.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Di Sarno A, Landi ML, Cappabianca P, et al. . Resistance to cabergoline as compared with bromocriptine in hyperprolactinemia: prevalence, clinical definition, and therapeutic strategy. J Clin Endocrinol Metab. 2001;86(11):5256-5261. - PubMed
-
- Delgrange E, Daems T, Verhelst J, Abs R, Maiter D. Characterization of resistance to the prolactin-lowering effects of cabergoline in macroprolactinomas: a study in 122 patients. Eur J Endocrinol. 2009;160:747-752. - PubMed
-
- Melmed S. Pituitary-tumor endocrinopathies. N Engl J Med. 2020;382:937-950. - PubMed
-
- Melmed S, Casanueva FF, Hoffman AR, et al. ; Endocrine Society . Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273-288. - PubMed
-
- Ioachimescu AG, Fleseriu M, Hoffman AR, Vaughan TB 3rd, Katznelson L. Psychological effects of dopamine agonist treatment in patients with hyperprolactinemia and prolactin-secreting adenomas. Eur J Endocrinol. 2019;180(1):31-40. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

