Prospective practice survey of management of cetuximab-related skin reactions
- PMID: 33150521
- PMCID: PMC8163687
- DOI: 10.1007/s00520-020-05862-7
Prospective practice survey of management of cetuximab-related skin reactions
Abstract
Purpose: Evidence-based guidelines on how to prevent or treat cetuximab-related skin reactions are lacking and multiple care and management strategies are used. The main purpose of the present study is to gain information about the different skincare products being used against skin reactions in metastatic colorectal cancer (mCRC) and recurrent/metastatic (R/M) or locally advanced (LA) squamous cell cancer of the head and neck (SCCHN) patients treated with cetuximab.
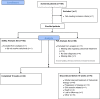
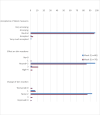
Methods: An open-label, prospective observational study conducted in the Netherlands. The occurrence of skin reactions and the care and management options taken were documented for 16 weeks, starting from the first administration of cetuximab.
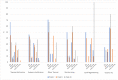
Results: A total of 103 patients were included in 7 hospitals. 38 patients (37%) developed a grade ≥ 2 skin reaction. Eighty-six patients could be analysed for the primary endpoint (73.3% males, mean age 62.4 years, n = 44 LA SCCHN, n = 16 R/M SCCHN, n = 26 mCRC). The most frequently used skin products at some point during the observation period were moisturizing products (70%), systemic antibiotics (64%), topical antibiotics (58%), lipid-regenerating (28%) and other topical products (28%). The overall use of products gradually increased from baseline to week 6-10, reducing by week 16. Hospital protocols were the primary reason (> 50%) for choice of the skincare products and medications.
Conclusion: A variety of skin care products and antibiotics were commonly used. Only few patients developed severe cutaneous reactions. For patients, the occurrence of skin reactions did not influence their willingness to continue cetuximab therapy.
Keywords: Cetuximab; Colorectal cancer; Head and neck cancer; Management; Skin reaction.
Conflict of interest statement
This study was sponsored by Merck B.V., Schiphol-Rijk, The Netherlands, an affiliate of Merck KGaA, Darmstadt, Germany. C. del Grande and B. van Doorn are employees of Merck B.V. The other authors do not have financial relationships with Merck B.V.
Figures
References
-
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. - DOI - PubMed
-
- Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, de Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical