Influences of Xeno-Free Media on Mesenchymal Stem Cell Expansion for Clinical Application
- PMID: 33150562
- PMCID: PMC7862486
- DOI: 10.1007/s13770-020-00306-z
Influences of Xeno-Free Media on Mesenchymal Stem Cell Expansion for Clinical Application
Abstract
Mesenchymal stem/stromal cells (MSCs) are multipotent somatic stem/progenitor cells that can be isolated from various tissues and have attracted increasing attention from the scientific community. This is due to MSCs showing great potential for incurable disease treatment, and most applications of MSCs involve tissue degeneration and treatment of immune- and inflammation-mediated diseases. Conventional MSC cultures contain fetal bovine serum (FBS), which is a common supplement for cell development but is also a risk factor for exposure to animal-derived pathogens. To avoid the risks resulting from the xenogeneic origin and animal-derived pathogens of FBS, xeno-free media have been developed and commercialized to satisfy MSC expansion demands for human clinical applications. This review summarized and provided an overview of xeno-free media that are currently used for MSC expansion. Additionally, we discussed the influences of different xeno-free media on MSC biology with particular regard to cell morphology, surface marker expression, proliferation, differentiation and immunomodulation. The xeno-free media can be serum-free and xeno-free media or media supplemented with some human-originating substances, such as human serum, human platelet lysates, human umbilical cord serum/plasma, or human plasma-derived supplements for cell culture medium. These media have capacity to maintain a spindle-shaped morphology, the expression of typical surface markers, and the capacity of multipotent differentiation and immunomodulation of MSCs. Xeno-free media showed potential for safe use for human clinical treatment. However, the influences of these xeno-free media on MSCs are various and any xeno-free medium should be examined prior to being used for MSC cultures.
Keywords: Clinical application; Mesenchymal stem cells; Xeno-free and serum-free media; Xeno-free media.
Conflict of interest statement
The authors have no conflicts to declare.
Figures
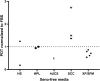
 ) indicates PDT of MSCs cultured in HS; black square (■) indicates PDT of MSCs cultured in HPL; black and white triangle (
) indicates PDT of MSCs cultured in HS; black square (■) indicates PDT of MSCs cultured in HPL; black and white triangle ( ) indicates PDT of MSCs cultured in hUCS; black and white square (
) indicates PDT of MSCs cultured in hUCS; black and white square ( ) indicates PDT of MSCs cultured in SCC; black diamond (◆) indicates PDT of MSCs cultured in XF/SFM
) indicates PDT of MSCs cultured in SCC; black diamond (◆) indicates PDT of MSCs cultured in XF/SFMSimilar articles
-
Differential Development of Umbilical Cord-Derived Mesenchymal Stem Cells During Long-Term Maintenance in Fetal Bovine Serum-Supplemented Medium and Xeno- and Serum-Free Culture Medium.Cell Reprogram. 2021 Dec;23(6):359-369. doi: 10.1089/cell.2021.0050. Epub 2021 Nov 8. Cell Reprogram. 2021. PMID: 34748399
-
A Three-Dimensional Xeno-Free Culture Condition for Wharton's Jelly-Mesenchymal Stem Cells: The Pros and Cons.Int J Mol Sci. 2023 Feb 13;24(4):3745. doi: 10.3390/ijms24043745. Int J Mol Sci. 2023. PMID: 36835154 Free PMC article.
-
Robust differentiation and potent immunomodulation of human mesenchymal stromal cells cultured with a xeno-free GMP protein supplement.Cytotherapy. 2025 Apr;27(4):552-561. doi: 10.1016/j.jcyt.2025.01.003. Epub 2025 Jan 11. Cytotherapy. 2025. PMID: 39864016
-
Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion.N Biotechnol. 2015 Jan 25;32(1):199-211. doi: 10.1016/j.nbt.2014.06.001. Epub 2014 Jun 11. N Biotechnol. 2015. PMID: 24929129 Free PMC article. Review.
-
Serum-Free Cultures: Could They Be a Future Direction to Improve Neuronal Differentiation of Mesenchymal Stromal Cells?Int J Mol Sci. 2022 Jun 7;23(12):6391. doi: 10.3390/ijms23126391. Int J Mol Sci. 2022. PMID: 35742836 Free PMC article. Review.
Cited by
-
Evaluation of Osteogenic Potential for Rat Adipose-Derived Stem Cells under Xeno-Free Environment.Int J Mol Sci. 2023 Dec 15;24(24):17532. doi: 10.3390/ijms242417532. Int J Mol Sci. 2023. PMID: 38139360 Free PMC article.
-
In vitro and in vivo assessment of a non-animal sourced chitosan scaffold loaded with xeno-free umbilical cord mesenchymal stromal cells cultured under macromolecular crowding conditions.Biomater Biosyst. 2024 Oct 10;16:100102. doi: 10.1016/j.bbiosy.2024.100102. eCollection 2024 Dec. Biomater Biosyst. 2024. PMID: 40225717 Free PMC article.
-
Optimization of Mesenchymal Stromal Cell (MSC) Manufacturing Processes for a Better Therapeutic Outcome.Front Immunol. 2022 Jun 9;13:918565. doi: 10.3389/fimmu.2022.918565. eCollection 2022. Front Immunol. 2022. PMID: 35812460 Free PMC article. Review.
-
A Three-Dimensional Printed Polycaprolactone-Biphasic-Calcium-Phosphate Scaffold Combined with Adipose-Derived Stem Cells Cultured in Xenogeneic Serum-Free Media for the Treatment of Bone Defects.J Funct Biomater. 2022 Jul 15;13(3):93. doi: 10.3390/jfb13030093. J Funct Biomater. 2022. PMID: 35893462 Free PMC article.
-
Platelet-rich fibrin-conditioned medium promotes osteogenesis of dental pulp stem cells through TGF-β and PDGF signaling.Regen Ther. 2025 May 27;30:100-106. doi: 10.1016/j.reth.2025.05.006. eCollection 2025 Dec. Regen Ther. 2025. PMID: 40511256 Free PMC article.
References
-
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
