Budget Impact of Oral Semaglutide Intensification versus Sitagliptin among US Patients with Type 2 Diabetes Mellitus Uncontrolled with Metformin
- PMID: 33150566
- PMCID: PMC7882575
- DOI: 10.1007/s40273-020-00967-7
Budget Impact of Oral Semaglutide Intensification versus Sitagliptin among US Patients with Type 2 Diabetes Mellitus Uncontrolled with Metformin
Abstract
Background: Oral semaglutide was approved in 2019 for blood glucose control in patients with type 2 diabetes mellitus (T2DM) and was the first oral glucagon-like peptide 1 receptor agonist (GLP-1 RA). T2DM is associated with substantial healthcare expenditures in the US, so the cost of a new intervention should be weighed against clinical benefits.
Objective: This study evaluated the budget impact of a treatment pathway with oral semaglutide 14 mg daily versus oral sitagliptin 100 mg daily among patients not achieving target glycated hemoglobin (HbA1c) level despite treatment with metformin.
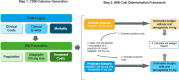
Methods: This study used the validated IQVIA™ CORE Diabetes Model to simulate the treatment impact of oral semaglutide 14 mg and sitagliptin 100 mg over a 5-year time horizon from a US healthcare sector (payer) perspective. Trial data (PIONEER 3) informed cohort characteristics and treatment effects, and literature sources informed event costs. Population and market share data were from the literature and data on file. The analysis evaluated the estimated budget impact of oral semaglutide 14 mg use for patients currently using sitagliptin 100 mg considering both direct medical and treatment costs to understand the impact on total cost of care, given underlying treatment performance and impact on avoidable events.
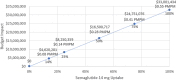
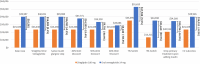
Results: In a hypothetical plan of 1 million lives, an estimated 1993 patients were treated with sitagliptin 100 mg in the target population. Following these patients over 5 years, the incremental direct medical and treatment costs of a patient using oral semaglutide 14 mg versus sitagliptin 100 mg was $US16,562, a 70.7% increase (year 2019 values). A hypothetical payer would spend an additional $US3,300,143 (7.1%) over 5 years for every 10% of market share that oral semaglutide 14 mg takes away from sitagliptin 100 mg. Univariate and scenario analyses with alternate inputs and assumptions demonstrated consistent results.
Conclusions: Use of oral semaglutide 14 mg in patients currently receiving sitagliptin 100 mg substantially increases the budget impact for patients with T2DM whose blood glucose level is not controlled with metformin over a 5-year time horizon for US healthcare payers.
Budget impact of oral semaglutide intensification versus sitagliptin among US patients with type 2 diabetes mellitus uncontrolled with metformin.
Plain language summary
Patients with type 2 diabetes mellitus (T2DM) have many treatment options. Choices depend on factors such as cost, preference, and patient characteristics. Oral semaglutide was recently approved for the treatment of T2DM as the first oral therapy of its class. This study estimated the cost for patients treated with sitagliptin 100 mg, a commonly used T2DM treatment, versus oral semaglutide 14 mg for patients whose disease is not well controlled with metformin. Costs and effects were estimated over 5 years for each treatment strategy using predictive model equations and clinical trial data for the two treatments. These costs were considered for both a hypothetical healthcare plan of 1 million lives and the full US population. A patient treated with oral semaglutide 14 mg would expect to see 70.7% higher costs than a patient treated with sitagliptin 100 mg over 5 years. For every 10% of patients who would switch from sitagliptin 100 mg to oral semaglutide 14 mg, costs would increase by 7.1%. Changing the cost of oral semaglutide 14 mg had the greatest impact on model results. The findings from the analysis were consistent across a range of alternate model inputs. Oral semaglutide 14 mg is more costly than sitagliptin 100 mg over 5 years.
Conflict of interest statement
Adnan Alsumali, Dominik Lautsch, and Swapnil Rajpathak are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Glenn Davies was an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, at the time of the study. Andrew Briggs is a director and shareholder of Avalon Health Economics and received compensation from Merck & Co., Inc. as a consultant for this work. He has also been contracted by Bayer, Eisai, Janssen, Novartis, Sword Health, Amgen, and Daichii Sankyo and received compensation outside of the submitted work. Elizabeth Wehler is an employee of IQVIA, a consulting company that received funding from Merck & Co., Inc. to conduct the study as well as from other companies. Qianyi Li and Stacey Kowal were employees of IQVIA at the time of the study.
Figures
Similar articles
-
Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated Hemoglobin in Adults With Type 2 Diabetes Uncontrolled With Metformin Alone or With Sulfonylurea: The PIONEER 3 Randomized Clinical Trial.JAMA. 2019 Apr 16;321(15):1466-1480. doi: 10.1001/jama.2019.2942. JAMA. 2019. PMID: 30903796 Free PMC article. Clinical Trial.
-
The Cost-Effectiveness of Oral Semaglutide in Spain: A Long-Term Health Economic Analysis Based on the PIONEER Clinical Trials.Adv Ther. 2022 Jul;39(7):3180-3198. doi: 10.1007/s12325-022-02156-8. Epub 2022 May 12. Adv Ther. 2022. PMID: 35553372
-
Cost-effectiveness of oral semaglutide added to current antihyperglycemic treatment for type 2 diabetes.J Manag Care Spec Pharm. 2021 Apr;27(4):455-468. doi: 10.18553/jmcp.2021.27.4.455. J Manag Care Spec Pharm. 2021. PMID: 33769850 Free PMC article.
-
Repaglinide : a pharmacoeconomic review of its use in type 2 diabetes mellitus.Pharmacoeconomics. 2004;22(6):389-411. doi: 10.2165/00019053-200422060-00005. Pharmacoeconomics. 2004. PMID: 15099124 Review.
-
Comparative efficacy and safety profile of once-weekly Semaglutide versus once-daily Sitagliptin as an add-on to metformin in patients with type 2 diabetes: a systematic review and meta-analysis.Ann Med. 2023;55(2):2239830. doi: 10.1080/07853890.2023.2239830. Ann Med. 2023. PMID: 37498865 Free PMC article.
Cited by
-
Budget Impact Analysis of Diabetes Drugs: A Systematic Literature Review.Front Public Health. 2021 Nov 19;9:765999. doi: 10.3389/fpubh.2021.765999. eCollection 2021. Front Public Health. 2021. PMID: 34869180 Free PMC article.
-
A novel effervescent formulation of oral weekly alendronate (70 mg) improves persistence compared to alendronate tablets in post-menopausal women with osteoporosis.Aging Clin Exp Res. 2021 Sep;33(9):2529-2537. doi: 10.1007/s40520-020-01777-9. Epub 2021 Jan 15. Aging Clin Exp Res. 2021. PMID: 33449337
-
Improving bone health: addressing the burden through an integrated approach.Aging Clin Exp Res. 2021 Oct;33(10):2777-2786. doi: 10.1007/s40520-021-01971-3. Epub 2021 Oct 6. Aging Clin Exp Res. 2021. PMID: 34613608
References
-
- American Diabetes Association. Facts About Type 2. https://www.diabetes.org/diabetes-basics/type-2/facts-about-type-2.html. Accessed May 2019.
-
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Estimates of Diabetes and Its Burden in the United States. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-stat.... Accessed Sept 2019.
-
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet (London, England) 2017;389(10082):1907–1918. doi: 10.1016/s0140-6736(17)30505-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

