A systematic review of reporting quality for anaesthetic interventions in randomised controlled trials
- PMID: 33150618
- PMCID: PMC8246731
- DOI: 10.1111/anae.15294
A systematic review of reporting quality for anaesthetic interventions in randomised controlled trials
Abstract
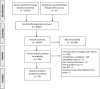
Interventions from randomised controlled trials can only be replicated if they are reported in sufficient detail. The results of trials can only be confidently interpreted if the delivery of the intervention was systematic and the protocol adhered to. We systematically reviewed trials of anaesthetic interventions published in 12 journals from January 2016 to September 2019. We assessed the detail with which interventions were reported, using the Consolidated Standards of Reporting Trials statement for non-pharmacological treatments. We analysed 162 interventions reported by 78 trials in 18,675 participants. Detail sufficiently precise to replicate the intervention was reported for 111 (69%) interventions. Intervention standardisation was reported for 135 (83%) out of the 162 interventions, and protocol adherence was reported for 20 (12%) interventions. Sixty (77%) out of the 78 trials reported the administrative context in which interventions were delivered and 36 (46%) trials detailed the expertise of the practitioners. We conclude that bespoke reporting tools should be developed for anaesthetic interventions and interventions in other areas such as critical care.
Keywords: adherence; protocols and guidelines; randomised controlled trials; reporting standards; standardisation.
© 2020 The Authors. Anaesthesia published by John Wiley & Sons Ltd on behalf of Association of Anaesthetists.
References
-
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Annals of Internal Medicine 2008; 148: 295–309. - PubMed
-
- Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P. CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Annals of Internal Medicine 2017; 167: 40–7. - PubMed
-
- Halpern SH, Darani R, Douglas MJ, Wight W, Yee J. Compliance with the CONSORT checklist in obstetric anaesthesia randomised controlled trials. International Journal of Obstetric Anesthesia 2004; 13: 207–14. - PubMed
-
- O’Donnell CM, McLoughlin L, Patterson CC, et al. Perioperative outcomes in the context of mode of anaesthesia for patients undergoing hip fracture surgery: systematic review and meta‐analysis. British Journal of Anaesthesia 2018; 120: 37–50. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical