Transcription factor old astrocyte specifically induced substance is a novel regulator of kidney fibrosis
- PMID: 33150680
- PMCID: PMC7821213
- DOI: 10.1096/fj.202001820R
Transcription factor old astrocyte specifically induced substance is a novel regulator of kidney fibrosis
Abstract
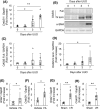
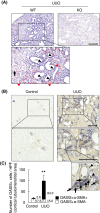
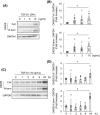
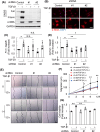
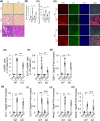
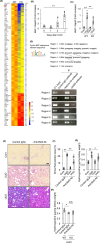
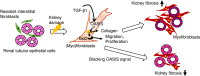
Prevention of kidney fibrosis is an essential requisite for effective therapy in preventing chronic kidney disease (CKD). Here, we identify Old astrocyte specifically induced substance (OASIS)/cAMP responsive element-binding protein 3-like 1 (CREB3l1), a CREB/ATF family transcription factor, as a candidate profibrotic gene that drives the final common pathological step along the fibrotic pathway in CKD. Although microarray data from diseased patient kidneys and fibrotic mouse model kidneys both exhibit OASIS/Creb3l1 upregulation, the pathophysiological roles of OASIS in CKD remains unknown. Immunohistochemistry revealed that OASIS protein was overexpressed in human fibrotic kidney compared with normal kidney. Moreover, OASIS was upregulated in murine fibrotic kidneys, following unilateral ureteral obstruction (UUO), resulting in an increase in the number of OASIS-expressing pathological myofibroblasts. In vitro assays revealed exogenous TGF-β1 increased OASIS expression coincident with fibroblast-to-myofibroblast transition and OASIS contributed to TGF-β1-mediated myofibroblast migration and increased proliferation. Significantly, in vivo kidney fibrosis induced via UUO or ischemia/reperfusion injury was ameliorated by systemic genetic knockout of OASIS, accompanied by reduced myofibroblast proliferation. Microarrays revealed that the transmembrane glycoprotein Bone marrow stromal antigen 2 (Bst2) expression was reduced in OASIS knockout myofibroblasts. Interestingly, a systemic anti-Bst2 blocking antibody approach attenuated kidney fibrosis in normal mice but not in OASIS knockout mice after UUO, signifying Bst2 functions downstream of OASIS. Finally, myofibroblast-restricted OASIS conditional knockouts resulted in resistance to kidney fibrosis. Taken together, OASIS in myofibroblasts promotes kidney fibrosis, at least in part, via increased Bst2 expression. Thus, we have identified and demonstrated that OASIS signaling is a novel regulator of kidney fibrosis.
Keywords: chronic kidney disease; fibrosis; myofibroblast; transcription regulation.
© 2020 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures



Similar articles
-
Upregulation of OASIS/CREB3L1 in podocytes contributes to the disturbance of kidney homeostasis.Commun Biol. 2022 Jul 22;5(1):734. doi: 10.1038/s42003-022-03709-x. Commun Biol. 2022. PMID: 35869269 Free PMC article.
-
Adenovirus-mediated P311 ameliorates renal fibrosis through inhibition of epithelial-mesenchymal transition via TGF-β1-Smad-ILK pathway in unilateral ureteral obstruction rats.Int J Mol Med. 2018 May;41(5):3015-3023. doi: 10.3892/ijmm.2018.3485. Epub 2018 Feb 12. Int J Mol Med. 2018. PMID: 29436600
-
Targeted delivery of type I TGF-β receptor-mimicking peptide to fibrotic kidney for improving kidney fibrosis therapy via enhancing the inhibition of TGF-β1/Smad and p38 MAPK pathways.Int Immunopharmacol. 2024 Aug 20;137:112483. doi: 10.1016/j.intimp.2024.112483. Epub 2024 Jun 15. Int Immunopharmacol. 2024. PMID: 38880023
-
CREB3L1/OASIS: cell cycle regulator and tumor suppressor.FEBS J. 2024 Nov;291(22):4853-4866. doi: 10.1111/febs.17052. Epub 2024 Jan 12. FEBS J. 2024. PMID: 38215153 Review.
-
The role of macrophages in fibrosis of chronic kidney disease.Biomed Pharmacother. 2024 Aug;177:117079. doi: 10.1016/j.biopha.2024.117079. Epub 2024 Jul 4. Biomed Pharmacother. 2024. PMID: 38968801 Review.
Cited by
-
Fatty acid metabolism suppresses neonatal cardiomyocyte proliferation by increasing PDK4 and HMGCS2 expression through PPARδ.PLoS One. 2025 May 8;20(5):e0318178. doi: 10.1371/journal.pone.0318178. eCollection 2025. PLoS One. 2025. PMID: 40338840 Free PMC article.
-
The Regulatory Network of CREB3L1 and Its Roles in Physiological and Pathological Conditions.Int J Med Sci. 2024 Jan 1;21(1):123-136. doi: 10.7150/ijms.90189. eCollection 2024. Int J Med Sci. 2024. PMID: 38164349 Free PMC article. Review.
-
CD317 stabilizes TNFR1 and confers the anti-inflammatory functions of MSCs via NF-κB/TSG6 pathway.Stem Cell Res Ther. 2025 Jul 21;16(1):391. doi: 10.1186/s13287-025-04527-8. Stem Cell Res Ther. 2025. PMID: 40691615 Free PMC article.
-
Single-cell multiomics reveals the complexity of TGFβ signalling to chromatin in iPSC-derived kidney organoids.Commun Biol. 2022 Nov 27;5(1):1301. doi: 10.1038/s42003-022-04264-1. Commun Biol. 2022. PMID: 36435939 Free PMC article.
-
CXCL10 is a novel anti-angiogenic factor downstream of p53 in cardiomyocytes.Physiol Rep. 2022 May;10(9):e15304. doi: 10.14814/phy2.15304. Physiol Rep. 2022. PMID: 35542987 Free PMC article.
References
-
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296‐1305. - PubMed
-
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow‐up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164(6):659‐663. - PubMed
-
- Moll S, Meier M, Formentini I, Pomposiello S, Prunotto M. New renal drug development to face chronic renal disease. Expert Opin Drug Discov. 2014;9(12):1471‐1485. - PubMed
-
- Strutz F, Zeisberg M. Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol. 2006;17(11):2992‐2998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

