A Human Vascular Injury-on-a-Chip Model of Hemostasis
- PMID: 33150735
- PMCID: PMC8049960
- DOI: 10.1002/smll.202004889
A Human Vascular Injury-on-a-Chip Model of Hemostasis
Abstract
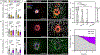
Hemostasis is an innate protective mechanism that plays a central role in maintaining the homeostasis of the vascular system during vascular injury. Studying this essential physiological process is often challenged by the difficulty of modeling and probing the complex dynamics of hemostatic responses in the native context of human blood vessels. To address this major challenge, this paper describes a microengineering approach for in vitro modeling of hemostasis. This microphysiological model replicates the living endothelium, multilayered microarchitecture, and procoagulant activity of human blood vessels, and is also equipped with a microneedle that is actuated with spatial precision to simulate penetrating vascular injuries. The system recapitulates key features of the hemostatic response to acute vascular injury as observed in vivo, including i) thrombin-driven accumulation of platelets and fibrin, ii) formation of a platelet- and fibrin-rich hemostatic plug that halts blood loss, and iii) matrix deformation driven by platelet contraction for wound closure. Moreover, the potential use of this model for drug testing applications is demonstrated by evaluating the effects of anticoagulants and antiplatelet agents that are in current clinical use. The vascular injury-on-a-chip may serve as an enabling platform for preclinical investigation of hematological disorders and emerging therapeutic approaches against them.
Keywords: fibrin; hemostasis; microphysiological system; platelets; vascular injury-on-a-chip.
© 2020 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interest
D.H. holds equity in Emulate Inc.
Figures




References
-
- Jackson SP, Nat Med 2011, 17, 1423–1436. - PubMed
-
- Stalker TJ, Welsh JD, Tomaiuolo M, Wu J, Colace TV, Diamond SL and Brass LF, Blood 2014, 124, 1824–1831; - PMC - PubMed
- Tomaiuolo M, Stalker TJ, Welsh JD, Diamond SL, Sinno T and Brass LF, Blood 2014, 124, 1816–1823; - PMC - PubMed
- Welsh JD, Stalker TJ, Voronov R, Muthard RW, Tomaiuolo M, Diamond SL and Brass LF, Blood 2014, 124, 1808–1815. - PMC - PubMed
-
- Jagadeeswaran P, Cooley BC, Gross PL and Mackman N, Circ Res 2016, 118, 1363–1379. - PubMed
-
- Greene TK, Schiviz A, Hoellriegl W, Poncz M, Muchitsch EM, Animal S Models Subcommittee of the and I. Standardization Committee Of The, J Thromb Haemost 2010, 8, 2820–2822. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

