Aldosterone enhances high phosphate-induced vascular calcification through inhibition of AMPK-mediated autophagy
- PMID: 33150736
- PMCID: PMC7754028
- DOI: 10.1111/jcmm.15813
Aldosterone enhances high phosphate-induced vascular calcification through inhibition of AMPK-mediated autophagy
Abstract
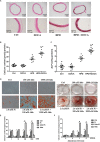
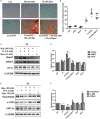
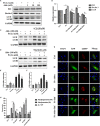
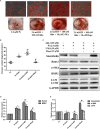
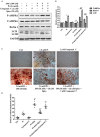
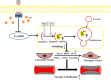
It remains unclear whether the necessity of calcified mellitus induced by high inorganic phosphate (Pi) is required and the roles of autophagy plays in aldosterone (Aldo)-enhanced vascular calcification (VC) and vascular smooth muscle cell (VSMC) osteogenic differentiation. In the present study, we found that Aldo enhanced VC both in vivo and in vitro only in the presence of high Pi, alongside with increased expression of VSMC osteogenic proteins (BMP2, Runx2 and OCN) and decreased expression of VSMC contractile proteins (α-SMA, SM22α and smoothelin). However, these effects were blocked by mineralocorticoid receptor inhibitor, spironolactone. In addition, the stimulatory effects of Aldo on VSMC calcification were further accelerated by the autophagy inhibitor, 3-MA, and were counteracted by the autophagy inducer, rapamycin. Moreover, inhibiting adenosine monophosphate-activated protein kinase (AMPK) by Compound C attenuated Aldo/MR-enhanced VC. These results suggested that Aldo facilitates high Pi-induced VSMC osteogenic phenotypic switch and calcification through MR-mediated signalling pathways that involve AMPK-dependent autophagy, which provided new insights into Aldo excess-associated VC in various settings.
Keywords: aldosterone; autophagy; phenotypic switch; vascular calcification; vascular smooth muscle cell.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures
Similar articles
-
Upregulated LncRNA H19 Sponges MiR-106a-5p and Contributes to Aldosterone-Induced Vascular Calcification via Activating the Runx2-Dependent Pathway.Arterioscler Thromb Vasc Biol. 2023 Sep;43(9):1684-1699. doi: 10.1161/ATVBAHA.123.319308. Epub 2023 Jul 6. Arterioscler Thromb Vasc Biol. 2023. PMID: 37409531
-
Death-associated protein kinase 3 deficiency alleviates vascular calcification via AMPK-mediated inhibition of endoplasmic reticulum stress.Eur J Pharmacol. 2019 Jun 5;852:90-98. doi: 10.1016/j.ejphar.2019.03.007. Epub 2019 Mar 7. Eur J Pharmacol. 2019. PMID: 30851272
-
Advanced glycation end-products suppress autophagy by AMPK/mTOR signaling pathway to promote vascular calcification.Mol Cell Biochem. 2020 Aug;471(1-2):91-100. doi: 10.1007/s11010-020-03769-9. Epub 2020 Jun 8. Mol Cell Biochem. 2020. PMID: 32514882
-
Roles of aldosterone in vascular calcification: An update.Eur J Pharmacol. 2016 Sep 5;786:186-193. doi: 10.1016/j.ejphar.2016.05.030. Epub 2016 May 26. Eur J Pharmacol. 2016. PMID: 27238972 Review.
-
Vascular Calcification-New Insights Into Its Mechanism.Int J Mol Sci. 2020 Apr 13;21(8):2685. doi: 10.3390/ijms21082685. Int J Mol Sci. 2020. PMID: 32294899 Free PMC article. Review.
Cited by
-
Effect of eplerenone on cognitive impairment in spontaneously hypertensive rats.Am J Transl Res. 2022 Jun 15;14(6):3864-3878. eCollection 2022. Am J Transl Res. 2022. PMID: 35836906 Free PMC article.
-
β-Hydroxybutyric Inhibits Vascular Calcification via Autophagy Enhancement in Models Induced by High Phosphate.Front Cardiovasc Med. 2021 Aug 20;8:685748. doi: 10.3389/fcvm.2021.685748. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34504876 Free PMC article.
-
Deletion of soluble epoxide hydrolase suppressed chronic kidney disease-related vascular calcification by restoring Sirtuin 3 expression.Cell Death Dis. 2021 Oct 23;12(11):992. doi: 10.1038/s41419-021-04283-6. Cell Death Dis. 2021. PMID: 34689162 Free PMC article.
-
The Role of Autophagy in Type 2 Diabetic Kidney Disease Management.Cells. 2023 Nov 23;12(23):2691. doi: 10.3390/cells12232691. Cells. 2023. PMID: 38067119 Free PMC article. Review.
-
Roles of Nuclear Receptors in Vascular Calcification.Int J Mol Sci. 2021 Jun 17;22(12):6491. doi: 10.3390/ijms22126491. Int J Mol Sci. 2021. PMID: 34204304 Free PMC article. Review.
References
-
- Liabeuf S, Okazaki H, Desjardins L, et al. Vascular calcification in chronic kidney disease: are biomarkers useful for probing the pathobiology and the health risks of this process in the clinical scenario? Nephrol Dial Transplant. 2014;29:1275‐1284. - PubMed
-
- Monticone S, D'Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2018;6:41‐50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

