A regimen with caplacizumab, immunosuppression, and plasma exchange prevents unfavorable outcomes in immune-mediated TTP
- PMID: 33150928
- PMCID: PMC7986049
- DOI: 10.1182/blood.2020008021
A regimen with caplacizumab, immunosuppression, and plasma exchange prevents unfavorable outcomes in immune-mediated TTP
Abstract
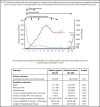
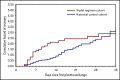
The anti-von Willebrand factor nanobody caplacizumab was licensed for adults with immune-mediated thrombotic thrombocytopenic purpura (iTTP) based on prospective controlled trials. However, few data are available on postmarketing surveillance. We treated 90 iTTP patients with a compassionate frontline triplet regimen associating therapeutic plasma exchange (TPE), immunosuppression with corticosteroids and rituximab, and caplacizumab. Outcomes were compared with 180 historical patients treated with the standard frontline treatment (TPE and corticosteroids, with rituximab as salvage therapy). The primary outcome was a composite of refractoriness and death within 30 days since diagnosis. Key secondary outcomes were exacerbations, time to platelet count recovery, the number of TPE, and the volume of plasma required to achieve durable remission. The percentage of patients in the triplet regimen with the composite primary outcome was 2.2% vs 12.2% in historical patients (P = .01). One elderly patient in the triplet regimen died of pulmonary embolism. Patients from this cohort experienced less exacerbations (3.4% vs 44%, P < .01); they recovered durable platelet count 1.8 times faster than historical patients (95% confidence interval, 1.41-2.36; P < .01), with fewer TPE sessions and lower plasma volumes (P < .01 both). The number of days in hospital was 41% lower in the triplet regimen than in the historical cohort (13 vs 22 days; P < .01). Caplacizumab-related adverse events occurred in 46 patients (51%), including 13 major or clinically relevant nonmajor hemorrhagic events. Associating caplacizumab to TPE and immunosuppression, by addressing the 3 processes of iTTP pathophysiology, prevents unfavorable outcomes and alleviates the burden of care.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: P.C. is member of the clinical advisory board for Alexion, Sanofi, Shire, and Octapharma. Y.B., P.P., A.W., Y.D., C.P., and A.V. participated in advisory boards for Sanofi. The remaining authors declare no competing financial interests.
Figures
Comment in
-
TTP: the evolution of clinical practice.Blood. 2021 Feb 11;137(6):719-720. doi: 10.1182/blood.2020009654. Blood. 2021. PMID: 33570615 No abstract available.
References
-
- Scully M, Cataland S, Coppo P, et al. ; International Working Group for Thrombotic Thrombocytopenic Purpura . Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15(2):312-322. - PubMed
-
- Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836-2846. - PubMed
-
- Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med. 1991;325(6):398-403. - PubMed
-
- Rock GA, Shumak KH, Buskard NA, et al. ; Canadian Apheresis Study Group . Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med. 1991;325(6):393-397. - PubMed
-
- Froissart A, Buffet M, Veyradier A, et al. ; Experience of the French Thrombotic Microangiopathies Reference Center . Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Crit Care Med. 2012;40(1):104-111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

