A New MRI Measure to Early Differentiate Progressive Supranuclear Palsy From De Novo Parkinson's Disease in Clinical Practice: An International Study
- PMID: 33151015
- PMCID: PMC8330364
- DOI: 10.1002/mds.28364
A New MRI Measure to Early Differentiate Progressive Supranuclear Palsy From De Novo Parkinson's Disease in Clinical Practice: An International Study
Abstract
Background: Enlargement of the third ventricle has been reported in atypical parkinsonism. We investigated whether the measurement of third ventricle width could distinguish Parkinson's disease (PD) from progressive supranuclear palsy (PSP).
Methods: We assessed a new MR T1-weighted measurement (third ventricle width/internal skull diameter) in a training cohort of 268 participants (98 PD, 73 PSP, 98 controls from our center) and in a testing cohort of 291 participants (82 de novo PD patients and 133 controls from the Parkinson's Progression Markers Initiative, 76 early-stage PSP from an international research group). PD diagnosis was confirmed after a 4-year follow-up. Diagnostic performance of the third ventricle/internal skull diameter was assessed using receiver operating characteristic curve with bootstrapping; the area under the curve of the training cohort was compared with the area under the curve of the testing cohort using the De Long test.
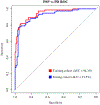
Results: In both cohorts, third ventricle/internal skull diameter values did not differ between PD and controls but were significantly lower in PD than in PSP patients (P < 0.0001). In PD, third ventricle/internal skull diameter values did not change significantly between baseline and follow-up evaluation. Receiver operating characteristic analysis accurately differentiated PD from PSP in the training cohort (area under the curve, 0.94; 95% CI, 91.1-97.6; cutoff, 5.72) and in the testing cohort (area under the curve, 0.91; 95% CI, 87.0-97.0; cutoff,: 5.88), validating the generalizability of the results.
Conclusion: Our study provides a new reliable and validated MRI measurement for the early differentiation of PD and PSP. The simplicity and generalizability of this biomarker make it suitable for routine clinical practice and for selection of patients in clinical trials worldwide. © 2020 International Parkinson and Movement Disorder Society.
Keywords: MRI biomarker; Parkinson's disease; clinical practice; progressive supranuclear palsy; third ventricle width.
© 2020 International Parkinson and Movement Disorder Society.
Conflict of interest statement
Figures

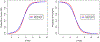
Comment in
-
Experience with a New Index to Differentiate Parkinson's Disease and Progressive Supranuclear Palsy.Mov Disord. 2021 Sep;36(9):2207-2208. doi: 10.1002/mds.28721. Mov Disord. 2021. PMID: 34543466 No abstract available.
-
Reply to: "Experience with a New Index to Differentiate Parkinson's Disease and Progressive Supranuclear Palsy".Mov Disord. 2021 Sep;36(9):2208-2209. doi: 10.1002/mds.28725. Mov Disord. 2021. PMID: 34543468 No abstract available.
References
-
- Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015;30:1591–1601. - PubMed
-
- Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology 2016;86:566–576. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

