Targeting Trimeric and Tetrameric Proanthocyanidins of Cinnamomum verum Bark as Bioactives for Dental Therapies
- PMID: 33151073
- PMCID: PMC8041212
- DOI: 10.1021/acs.jnatprod.0c00570
Targeting Trimeric and Tetrameric Proanthocyanidins of Cinnamomum verum Bark as Bioactives for Dental Therapies
Abstract
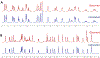
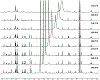
The present study elucidated the structures of three A-type tri- and tetrameric proanthocyanidins (PACs) isolated from Cinnamomum verum bark to the level of absolute configuration and determined their dental bioactivity using two therapeutically relevant bioassays. After selecting a PAC oligomer fraction via a biologically diverse bioassay-guided process, in tandem with centrifugal partition chromatography, phytochemical studies led to the isolation of PAC oligomers that represent the main bioactive principles of C. verum: two A-type tetrameric PACs, epicatechin-(2β→O→7,4β→8)-epicatechin-(4β→6)-epicatechin-(2β→O→7,4β→8)-catechin (1) and parameritannin A1 (2), together with a trimer, cinnamtannin B1 (3). Structure determination of the underivatized proanthocyanidins utilized a combination of HRESIMS, ECD, 1D/2D NMR, and 1H iterative full spin analysis data and led to NMR-based evidence for the deduction of absolute configuration in constituent catechin and epicatechin monomeric units.
Figures




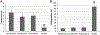
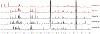
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

