Gefitinib induces EGFR and α5β1 integrin co-endocytosis in glioblastoma cells
- PMID: 33151388
- PMCID: PMC11073190
- DOI: 10.1007/s00018-020-03686-6
Gefitinib induces EGFR and α5β1 integrin co-endocytosis in glioblastoma cells
Abstract
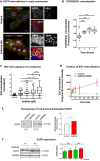
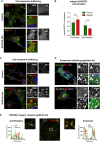
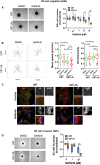
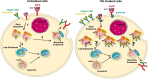
Overexpression of EGFR drives glioblastomas (GBM) cell invasion but these tumours remain resistant to EGFR-targeted therapies such as tyrosine kinase inhibitors (TKIs). Endocytosis, an important modulator of EGFR function, is often dysregulated in glioma cells and is associated with therapy resistance. However, the impact of TKIs on EGFR endocytosis has never been examined in GBM cells. In the present study, we showed that gefitinib and other tyrosine kinase inhibitors induced EGFR accumulation in early-endosomes as a result of an increased endocytosis. Moreover, TKIs trigger early-endosome re-localization of another membrane receptor, the fibronectin receptor alpha5beta1 integrin, a promising therapeutic target in GBM that regulates physiological EGFR endocytosis and recycling in cancer cells. Super-resolution dSTORM imaging showed a close-proximity between beta1 integrin and EGFR in intracellular membrane compartments of gefitinib-treated cells, suggesting their potential interaction. Interestingly, integrin depletion delayed gefitinib-mediated EGFR endocytosis. Co-endocytosis of EGFR and alpha5beta1 integrin may alter glioma cell response to gefitinib. Using an in vitro model of glioma cell dissemination from spheroid, we showed that alpha5 integrin-depleted cells were more sensitive to TKIs than alpha5-expressing cells. This work provides evidence for the first time that EGFR TKIs can trigger massive EGFR and alpha5beta1 integrin co-endocytosis, which may modulate glioma cell invasiveness under therapeutic treatment.
Keywords: Adhesion receptors; Brain cancer; Cell migration; Growth factors receptors; Membrane trafficking.
Conflict of interest statement
The authors disclose no conflicts.
Figures

Similar articles
-
Role of Endocytosis Proteins in Gefitinib-Mediated EGFR Internalisation in Glioma Cells.Cells. 2021 Nov 21;10(11):3258. doi: 10.3390/cells10113258. Cells. 2021. PMID: 34831480 Free PMC article.
-
The EGFR inhibitor gefitinib suppresses ligand-stimulated endocytosis of EGFR via the early/late endocytic pathway in non-small cell lung cancer cell lines.Histochem Cell Biol. 2007 May;127(5):541-53. doi: 10.1007/s00418-007-0281-y. Epub 2007 Mar 15. Histochem Cell Biol. 2007. PMID: 17361439
-
Evidence for efficient phosphorylation of EGFR and rapid endocytosis of phosphorylated EGFR via the early/late endocytic pathway in a gefitinib-sensitive non-small cell lung cancer cell line.Mol Cancer. 2008 May 21;7:42. doi: 10.1186/1476-4598-7-42. Mol Cancer. 2008. PMID: 18492291 Free PMC article.
-
EGFR, the Lazarus target for precision oncology in glioblastoma.Neuro Oncol. 2022 Dec 1;24(12):2035-2062. doi: 10.1093/neuonc/noac204. Neuro Oncol. 2022. PMID: 36125064 Free PMC article. Review.
-
Emerging functions of the EGFR in cancer.Mol Oncol. 2018 Jan;12(1):3-20. doi: 10.1002/1878-0261.12155. Epub 2017 Nov 27. Mol Oncol. 2018. PMID: 29124875 Free PMC article. Review.
Cited by
-
LncRNA NEAT1 Enhances Glioma Progression via Regulating the miR-128-3p/ITGA5 Axis.Mol Neurobiol. 2021 Oct;58(10):5163-5177. doi: 10.1007/s12035-021-02474-y. Epub 2021 Jul 15. Mol Neurobiol. 2021. PMID: 34263426 Free PMC article.
-
The complex relationship between integrins and oncolytic herpes Simplex Virus 1 in high-grade glioma therapeutics.Mol Ther Oncolytics. 2022 Jun 6;26:63-75. doi: 10.1016/j.omto.2022.05.013. eCollection 2022 Sep 15. Mol Ther Oncolytics. 2022. PMID: 35795093 Free PMC article. Review.
-
Role of Endocytosis Proteins in Gefitinib-Mediated EGFR Internalisation in Glioma Cells.Cells. 2021 Nov 21;10(11):3258. doi: 10.3390/cells10113258. Cells. 2021. PMID: 34831480 Free PMC article.
-
Glioblastoma multiforme: Diagnosis, treatment, and invasion.J Biomed Res. 2022 Oct 28;37(1):47-58. doi: 10.7555/JBR.36.20220156. J Biomed Res. 2022. PMID: 36403983 Free PMC article.
-
Bioimaging Nucleic-Acid Aptamers with Different Specificities in Human Glioblastoma Tissues Highlights Tumoral Heterogeneity.Pharmaceutics. 2022 Sep 20;14(10):1980. doi: 10.3390/pharmaceutics14101980. Pharmaceutics. 2022. PMID: 36297416 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

