Genome Mining and Metabolomics Uncover a Rare d-Capreomycidine Containing Natural Product and Its Biosynthetic Gene Cluster
- PMID: 33151679
- PMCID: PMC7830813
- DOI: 10.1021/acschembio.0c00663
Genome Mining and Metabolomics Uncover a Rare d-Capreomycidine Containing Natural Product and Its Biosynthetic Gene Cluster
Abstract
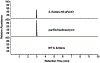
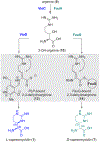
We report the metabolomics-driven genome mining of a new cyclic-guanidino incorporating non-ribosomal peptide synthetase (NRPS) gene cluster and full structure elucidation of its associated hexapeptide product, faulknamycin. Structural studies unveiled that this natural product contained the previously unknown (R,S)-stereoisomer of capreomycidine, d-capreomycidine. Furthermore, heterologous expression of the identified gene cluster successfully reproduces faulknamycin production without an observed homologue of VioD, the pyridoxal phosphate (PLP)-dependent enzyme found in all previous l-capreomycidine biosynthesis. An alternative NRPS-dependent pathway for d-capreomycidine biosynthesis is proposed.
Conflict of interest statement
The authors declare the following competing financial interest(s): W.W.M., N.L.K., and R.J.T, declare competing financial interests in MicroMGx, Inc.
Figures
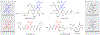


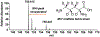
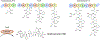
References
-
- Melo MJ (2009) History of Natural Dyes in the Ancient Mediterranean World. Handbook of Natural Colorants, 1–20.
-
- Katz L, and Baltz RH (2016) Natural product discovery: past, present, and future. J. Ind. Microbiol. Biotechnol 43 (2–3), 155–176. - PubMed
-
- Bérdy J (2005) Bioactive Microbial Metabolites. J. Antibiot 58 (1), 1–26. - PubMed
-
- Baltz RH (2006) Marcel Faber Roundtable: Is our antibiotic pipeline unproductive because of starvation, constipation or lack of inspiration? J. Ind. Microbiol. Biotechnol 33 (7), 507–513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

