Yap activation in irradiated parotid salivary glands is regulated by ROCK activity
- PMID: 33151927
- PMCID: PMC7644026
- DOI: 10.1371/journal.pone.0232921
Yap activation in irradiated parotid salivary glands is regulated by ROCK activity
Abstract
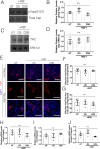
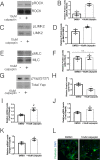
Radiotherapy plays a major role in the curative treatment of head and neck cancer, either as a single modality therapy, or in combination with surgery or chemotherapy, or both. Despite advances to limit radiation-induced side-effects, the major salivary glands are often affected. This frequently leads to hyposalivation which causes an increased risk for xerostomia, dental caries, mucositis, and malnutrition culminating in a significant impact on patients' quality of life. Previous research demonstrated that loss of salivary function is associated with a decrease in polarity regulators and an increase in nuclear Yap localization in a putative stem and progenitor cell (SPC) population. Yap activation has been shown to be essential for regeneration in intestinal injury models; however, the highest levels of nuclear Yap are observed in irradiated salivary SPCs that do not regenerate the gland. Thus, elucidating the inputs that regulate nuclear Yap localization and determining the role that Yap plays within the entire tissue following radiation damage and during regeneration is critical. In this study, we demonstrate that radiation treatment increases nuclear Yap localization in acinar cells and Yap-regulated genes in parotid salivary tissues. Conversely, administration of insulin-like growth factor 1 (IGF1), known to restore salivary function in mouse models, reduces nuclear Yap localization and Yap transcriptional targets to levels similar to untreated tissues. Activation of Rho-associated protein kinase (ROCK) using calpeptin results in increased Yap-regulated genes in primary acinar cells while inhibition of ROCK activity (Y-27632) leads to decreased Yap transcriptional targets. These results suggest that Yap activity is dependent on ROCK activity and provides new mechanistic insights into the regulation of radiation-induced hyposalivation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Persistent disruption of lateral junctional complexes and actin cytoskeleton in parotid salivary glands following radiation treatment.Am J Physiol Regul Integr Comp Physiol. 2018 Oct 1;315(4):R656-R667. doi: 10.1152/ajpregu.00388.2017. Epub 2018 Jun 13. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 29897817 Free PMC article.
-
Insulin-Like Growth Factor-1-Mediated DNA Repair in Irradiated Salivary Glands Is Sirtuin-1 Dependent.J Dent Res. 2017 Feb;96(2):225-232. doi: 10.1177/0022034516677529. Epub 2016 Nov 16. J Dent Res. 2017. PMID: 28106504 Free PMC article.
-
Administration of growth factors promotes salisphere formation from irradiated parotid salivary glands.PLoS One. 2018 Mar 28;13(3):e0193942. doi: 10.1371/journal.pone.0193942. eCollection 2018. PLoS One. 2018. PMID: 29590144 Free PMC article.
-
Pathophysiology and management of radiation-induced xerostomia.J Support Oncol. 2005 May-Jun;3(3):191-200. J Support Oncol. 2005. PMID: 15915820 Review.
-
Stem cells and the repair of radiation-induced salivary gland damage.Oral Dis. 2011 Mar;17(2):143-53. doi: 10.1111/j.1601-0825.2010.01723.x. Epub 2010 Aug 27. Oral Dis. 2011. PMID: 20796229 Review.
Cited by
-
Autologous mesenchymal stem cells offer a new paradigm for salivary gland regeneration.Int J Oral Sci. 2023 May 10;15(1):18. doi: 10.1038/s41368-023-00224-5. Int J Oral Sci. 2023. PMID: 37165024 Free PMC article. Review.
-
Salivary gland function, development, and regeneration.Physiol Rev. 2022 Jul 1;102(3):1495-1552. doi: 10.1152/physrev.00015.2021. Epub 2022 Mar 28. Physiol Rev. 2022. PMID: 35343828 Free PMC article. Review.
-
Peristalsis-Associated Mechanotransduction Drives Malignant Progression of Colorectal Cancer.Cell Mol Bioeng. 2023 Aug 11;16(4):261-281. doi: 10.1007/s12195-023-00776-w. eCollection 2023 Aug. Cell Mol Bioeng. 2023. PMID: 37811008 Free PMC article.
-
FAK Shutdown: Consequences on Epithelial Morphogenesis and Biomarker Expression Involving an Innovative Biomaterial for Tissue Regeneration.Int J Mol Sci. 2021 Sep 10;22(18):9774. doi: 10.3390/ijms22189774. Int J Mol Sci. 2021. PMID: 34575938 Free PMC article.
-
Radiation-Induced Salivary Gland Dysfunction: Mechanisms, Therapeutics and Future Directions.J Clin Med. 2020 Dec 18;9(12):4095. doi: 10.3390/jcm9124095. J Clin Med. 2020. PMID: 33353023 Free PMC article. Review.
References
-
- Buglione M., et al., Oral toxicity management in head and neck cancer patients treated with chemotherapy and radiation: Dental pathologies and osteoradionecrosis (Part 1) literature review and consensus statement. Crit Rev Oncol Hematol, 2016. 97: p. 131–42. 10.1016/j.critrevonc.2015.08.010 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

