Preliminary evaluations of 3-dimensional human skin models for their ability to facilitate in vitro the long-term development of the debilitating obligatory human parasite Onchocerca volvulus
- PMID: 33151944
- PMCID: PMC7671495
- DOI: 10.1371/journal.pntd.0008503
Preliminary evaluations of 3-dimensional human skin models for their ability to facilitate in vitro the long-term development of the debilitating obligatory human parasite Onchocerca volvulus
Abstract
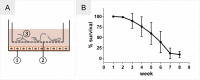
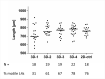
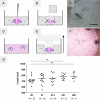
Onchocerciasis also known as river blindness is a neglected tropical disease and the world's second-leading infectious cause of blindness in humans; it is caused by Onchocerca volvulus. Current treatment with ivermectin targets microfilariae and transmission and does not kill the adult parasites, which reside within subcutaneous nodules. To support the development of macrofilaricidal drugs that target the adult worm to further support the elimination of onchocerciasis, an in-depth understanding of O. volvulus biology especially the factors that support the longevity of these worms in the human host (>10 years) is required. However, research is hampered by a lack of access to adult worms. O. volvulus is an obligatory human parasite and no small animal models that can propagate this parasite were successfully developed. The current optimized 2-dimensional (2-D) in vitro culturing method starting with O. volvulus infective larvae does not yet support the development of mature adult worms. To overcome these limitations, we have developed and applied 3-dimensional (3-D) culture systems with O. volvulus larvae that simulate the human in vivo niche using in vitro engineered skin and adipose tissue. Our proof of concept studies have shown that an optimized indirect co-culture of in vitro skin tissue supported a significant increase in growth of the fourth-stage larvae to the pre-adult stage with a median length of 816-831 μm as compared to 767 μm of 2-D cultured larvae. Notably, when larvae were co-cultured directly with adipose tissue models, a significant improvement for larval motility and thus fitness was observed; 95% compared to 26% in the 2-D system. These promising co-culture concepts are a first step to further optimize the culturing conditions and improve the long-term development of adult worms in vitro. Ultimately, it could provide the filarial research community with a valuable source of O. volvulus worms at various developmental stages, which may accelerate innovative unsolved biomedical inquiries into the parasite's biology.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Establishment of an in vitro culture system to study the developmental biology of Onchocerca volvulus with implications for anti-Onchocerca drug discovery and screening.PLoS Negl Trop Dis. 2021 Feb 9;15(2):e0008513. doi: 10.1371/journal.pntd.0008513. eCollection 2021 Feb. PLoS Negl Trop Dis. 2021. PMID: 33561123 Free PMC article.
-
Development of a preliminary in vitro drug screening assay based on a newly established culturing system for pre-adult fifth-stage Onchocerca volvulus worms.PLoS Negl Trop Dis. 2019 Jan 17;13(1):e0007108. doi: 10.1371/journal.pntd.0007108. eCollection 2019 Jan. PLoS Negl Trop Dis. 2019. PMID: 30653499 Free PMC article.
-
Onchocerca volvulus-specific antibody and cellular responses in onchocerciasis patients treated annually with ivermectin for 30 years and exposed to parasite transmission in central Togo.PLoS Negl Trop Dis. 2022 May 3;16(5):e0010340. doi: 10.1371/journal.pntd.0010340. eCollection 2022 May. PLoS Negl Trop Dis. 2022. PMID: 35503786 Free PMC article.
-
Development of emodepside as a possible adulticidal treatment for human onchocerciasis-The fruit of a successful industrial-academic collaboration.PLoS Pathog. 2021 Jul 22;17(7):e1009682. doi: 10.1371/journal.ppat.1009682. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34293063 Free PMC article. Review.
-
Onchocerca volvulus: The Road from Basic Biology to a Vaccine.Trends Parasitol. 2018 Jan;34(1):64-79. doi: 10.1016/j.pt.2017.08.011. Epub 2017 Sep 22. Trends Parasitol. 2018. PMID: 28958602 Free PMC article. Review.
Cited by
-
Establishment of an in vitro culture system to study the developmental biology of Onchocerca volvulus with implications for anti-Onchocerca drug discovery and screening.PLoS Negl Trop Dis. 2021 Feb 9;15(2):e0008513. doi: 10.1371/journal.pntd.0008513. eCollection 2021 Feb. PLoS Negl Trop Dis. 2021. PMID: 33561123 Free PMC article.
-
A mouse infection model and long-term lymphatic endothelium co-culture system to evaluate drugs against adult Brugia malayi.PLoS Negl Trop Dis. 2022 Jun 7;16(6):e0010474. doi: 10.1371/journal.pntd.0010474. eCollection 2022 Jun. PLoS Negl Trop Dis. 2022. PMID: 35671324 Free PMC article.
-
High-content approaches to anthelmintic drug screening.Trends Parasitol. 2021 Sep;37(9):780-789. doi: 10.1016/j.pt.2021.05.004. Epub 2021 Jun 3. Trends Parasitol. 2021. PMID: 34092518 Free PMC article. Review.
-
ReBiA-Robotic Enabled Biological Automation: 3D Epithelial Tissue Production.Adv Sci (Weinh). 2024 Dec;11(45):e2406608. doi: 10.1002/advs.202406608. Epub 2024 Sep 26. Adv Sci (Weinh). 2024. PMID: 39324843 Free PMC article.
-
Leveraging microphysiological systems to expedite understanding of host-parasite interactions.PLoS Pathog. 2025 Apr 24;21(4):e1013088. doi: 10.1371/journal.ppat.1013088. eCollection 2025 Apr. PLoS Pathog. 2025. PMID: 40273176 Free PMC article. Review.
References
-
- Kim YE, Remme JHF, Steinmann P, Stolk WA, Roungou J-B, Tediosi F. Control, Elimination, and Eradication of River Blindness: Scenarios, Timelines, and Ivermectin Treatment Needs in Africa. Lammie PJ, editor. PLoS Negl Trop Dis [Internet]. 2015. April 10;9(4):e0003664 Available from: 10.1371/journal.pntd.0003664 - DOI - PMC - PubMed
-
- NTD Modelling Consortium Onchocerciasis Group. The World Health Organization 2030 goals for onchocerciasis: Insights and perspectives from mathematical modelling: NTD Modelling Consortium Onchocerciasis Group. Gates open Res [Internet]. 2019. September 26;3:1545 Available from: https://gatesopenresearch.org/articles/3-1545/v1 10.12688/gatesopenres.13067.1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

