Immunological detection of pyrazine-2-carboxylic acid for the detection of pyrazinamide resistance in Mycobacterium tuberculosis
- PMID: 33151985
- PMCID: PMC7643994
- DOI: 10.1371/journal.pone.0241600
Immunological detection of pyrazine-2-carboxylic acid for the detection of pyrazinamide resistance in Mycobacterium tuberculosis
Erratum in
-
Correction: Immunological detection of pyrazine-2-carboxylic acid for the detection of pyrazinamide resistance in Mycobacterium tuberculosis.PLoS One. 2021 Oct 27;16(10):e0259439. doi: 10.1371/journal.pone.0259439. eCollection 2021. PLoS One. 2021. PMID: 34705897 Free PMC article.
Abstract
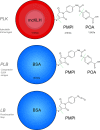
Pyrazinamide (PZA) susceptibility testing in Mycobacterium tuberculosis (Mtb) is a current area of development and PZA-resistant strains are increasingly prevalent. Previous studies have demonstrated that the detection of pyrazinoic acid (POA), the metabolite produced by the deamidation of PZA, is a good predictor for PZA resistance since a resistant strain would not convert PZA into POA at a critical required rate, whereas a susceptible strain will do, expelling POA to the extracellular environment at a certain rate, and allowing for quantification of this accumulated analyte. In order to quantify POA, an indirect competitive ELISA (icELISA) test using hyperimmune polyclonal rabbit serum against POA was developed: for this purpose, pure POA was first covalently linked to the highly immunogenic Keyhole Limpet Hemocyanine, and inoculated in rabbits. A construct made of bovine serum albumin (BSA) linked to pure POA and fixed at the bottom of wells was used as a competitor against spiked samples and liquid Mtb culture supernatants. When spiked samples (commercial POA alone) were analyzed, the half maximal inhibitory concentration (IC50) was 1.16 mg/mL, the limit of detection 200 μg/mL and the assay was specific (it did not detect PZA, IC50 > 20 mg/mL). However, culture supernatants (7H9-OADC-PANTA medium) disrupted the competition and a proper icELISA curve was not obtainable. We consider that, although we have shown that it is feasible to induce antibodies against POA, matrix effects could damage its analytical usefulness; multiple, upcoming ways to solve this obstacle are suggested.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- WHO. Global Tuberculosis Report. Geneva: World Health Organization, 2018.
-
- Girum T, Muktar E, Lentiro K, Wondiye H, Shewangizaw M. Epidemiology of multidrug-resistant tuberculosis (MDR-TB) in Ethiopia: a systematic review and meta-analysis of the prevalence, determinants and treatment outcome. Trop Dis Travel Med Vaccines. 2018;4:5 Epub 2018/06/27. 10.1186/s40794-018-0065-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

