Efficacy and safety of short-term therapy with indigo naturalis for ulcerative colitis: An investigator-initiated multicenter double-blind clinical trial
- PMID: 33151988
- PMCID: PMC7644062
- DOI: 10.1371/journal.pone.0241337
Efficacy and safety of short-term therapy with indigo naturalis for ulcerative colitis: An investigator-initiated multicenter double-blind clinical trial
Abstract
Introduction: Indigo naturalis (IN) is a blue pigment extracted from Assam indigo and other plants and has been confirmed to be highly effective for ulcerative colitis (UC) treatment in several clinical studies.
Objective: We conducted a multicenter double-blind study to confirm the efficacy and safety of short-term IN administration.
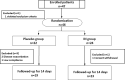
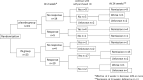
Methods: A multicenter, randomized controlled trial was conducted between December 2015 and October 2018 in our facilities. Forty-six patients with mild to moderate active UC (Lichtiger index: 5-10) were randomly assigned to the IN group or the placebo group and received 5 capsules (500 mg) twice a day for 2 weeks. We investigated the efficacy according to blood tests and the Lichtiger index before and after administration, and we also examined adverse events.
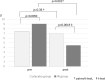
Results: The analysis included 42 patients (20 males, 22 females) with an average age of 45 years. Nineteen patients were assigned to the placebo group, and 23 were assigned to the IN group. After treatment administration, in the placebo group, no change in the Lichtiger index was observed (7.47 to 6.95, p = 0.359), and hemoglobin was significantly reduced (12.7 to 12.4, p = 0.031), while in the IN group, the Lichtiger index (9.04 to 4.48, p = 0.001) and albumin (4.0 to 4.12, p = 0.022) improved significantly. Mild headaches were observed in 5 patients and 1 patient in the IN and placebo groups, respectively.
Conclusions: Short-term administration of IN is highly effective without serious adverse events such as pulmonary hypertension or intussusception and may prevent the occurrence of serious adverse events.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

