Scientific quality of COVID-19 and SARS CoV-2 publications in the highest impact medical journals during the early phase of the pandemic: A case control study
- PMID: 33152034
- PMCID: PMC7643945
- DOI: 10.1371/journal.pone.0241826
Scientific quality of COVID-19 and SARS CoV-2 publications in the highest impact medical journals during the early phase of the pandemic: A case control study
Erratum in
-
Correction: Scientific quality of COVID-19 and SARS CoV-2 publications in the highest impact medical journals during the early phase of the pandemic: A case control study.PLoS One. 2021 Apr 8;16(4):e0250141. doi: 10.1371/journal.pone.0250141. eCollection 2021. PLoS One. 2021. PMID: 33831113 Free PMC article.
Abstract
Background: A debate about the scientific quality of COVID-19 themed research has emerged. We explored whether the quality of evidence of COVID-19 publications is lower when compared to nonCOVID-19 publications in the three highest ranked scientific medical journals.
Methods: We searched the PubMed Database from March 12 to April 12, 2020 and identified 559 publications in the New England Journal of Medicine, the Journal of the American Medical Association, and The Lancet which were divided into COVID-19 (cases, n = 204) and nonCOVID-19 (controls, n = 355) associated content. After exclusion of secondary, unauthored, response letters and non-matching article types, 155 COVID-19 publications (including 13 original articles) and 130 nonCOVID-19 publications (including 52 original articles) were included in the comparative analysis. The hierarchical level of evidence was determined for each publication included and compared between cases and controls as the main outcome. A quantitative scoring of quality was carried out for the subgroup of original articles. The numbers of authors and citation rates were also compared between groups.
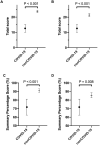
Results: The 130 nonCOVID-19 publications were associated with higher levels of evidence on the level of evidence pyramid, with a strong association measure (Cramer's V: 0.452, P <0.001). The 155 COVID-19 publications were 186-fold more likely to be of lower evidence (95% confidence interval [CI] for odds ratio, 7.0-47; P <0.001). The quantitative quality score (maximum possible score, 28) was significantly different in favor of nonCOVID-19 (mean difference, 11.1; 95% CI, 8.5-13.7; P <0.001). There was a significant difference in the early citation rate of the original articles that favored the COVID-19 original articles (median [interquartile range], 45 [30-244] vs. 2 [1-4] citations; P <0.001).
Conclusions: We conclude that the quality of COVID-19 publications in the three highest ranked scientific medical journals is below the quality average of these journals. These findings need to be verified at a later stage of the pandemic.
Conflict of interest statement
Marko Zdravkovic, Bogdan Zdravkovic and Joana Berger-Estilita have declared that no competing interests exist. David Berger has read the journal’s policy and the authors of this manuscript have the following competing interests: The Department of Intensive Care Medicine at Inselspital has, or has had in the past, research contracts with Abionic SA, AVA AG, CSEM SA, Cube Dx GmbH, Cyto Sorbents Europe GmbH, Edwards Lifesciences LLC, GE Healthcare, ImaCor Inc., MedImmune LLC, Orion Corporation, Phagenesis Ltd. and research & development/consulting contracts with Edwards Lifesciences LLC, Nestec SA, Wyss Zurich. The money was paid into a departmental fund; Dr Berger received no personal financial gain. The Department of Intensive Care Medicine has received unrestricted educational grants from the following organizations for organizing a quarterly postgraduate educational symposium, the Berner Forum for Intensive Care (until 2015): Abbott AG, Anandic Medical Systems, Astellas, AstraZeneca, Bard Medica SA, Baxter, B | Braun, CSL Behring, Covidien, Fresenius Kabi, GSK, Lilly, Maquet, MSD, Novartis, Nycomed, Orion Pharma, Pfizer, Pierre Fabre Pharma AG (formerly known as RobaPharm). The Department of Intensive Care Medicine has received unrestricted educational grants from the following organizations for organizing bi-annual postgraduate courses in the fields of critical care ultrasound, management of ECMO and mechanical ventilation: Abbott AG, Anandic Medical Systems, Bard Medica SA., Bracco, Dräger Schweiz AG, Edwards Lifesciences AG, Fresenius Kabi (Schweiz) AG, Getinge Group Maquet AG, Hamilton Medical AG, Pierre Fabre Pharma AG (formerly known as RobaPharm), PanGas AG Healthcare, Pfizer AG, Orion Pharma, Teleflex Medical GmbH. here are no patents, products in development or marketed products associated with this research to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

Similar articles
-
The wave of "opinion articles" in the coverage of COVID-19 in surgical literature.Langenbecks Arch Surg. 2020 Sep;405(6):877-878. doi: 10.1007/s00423-020-01932-w. Epub 2020 Jul 17. Langenbecks Arch Surg. 2020. PMID: 32676739 Free PMC article.
-
Impact of the COVID-19 pandemic on publication dynamics and non-COVID-19 research production.BMC Med Res Methodol. 2021 Nov 22;21(1):255. doi: 10.1186/s12874-021-01404-9. BMC Med Res Methodol. 2021. PMID: 34809561 Free PMC article.
-
Publishing in pandemic times: A bibliometric analysis of early medical publications on Kawasaki-like disease (MIS-C, PIMS-TS) related to SARS-CoV-2.Arch Pediatr. 2021 Aug;28(6):464-469. doi: 10.1016/j.arcped.2021.05.002. Epub 2021 May 28. Arch Pediatr. 2021. PMID: 34140220
-
[An analysis of global research on SARS-CoV-2].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2020 Apr 25;37(2):236-245. doi: 10.7507/1001-5515.202002034. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2020. PMID: 32329275 Free PMC article. Review. Chinese.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Sep 30;9:CD013639. doi: 10.1002/14651858.CD013639.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. PMID: 32997361 Updated.
Cited by
-
A Hierarchical Framework for Assessing Transmission Causality of Respiratory Viruses.Viruses. 2022 Jul 22;14(8):1605. doi: 10.3390/v14081605. Viruses. 2022. PMID: 35893670 Free PMC article. Review.
-
Comparison of citation rates between Covid-19 and non-Covid-19 articles across 24 major scientific journals.PLoS One. 2022 Jul 27;17(7):e0271071. doi: 10.1371/journal.pone.0271071. eCollection 2022. PLoS One. 2022. PMID: 35895698 Free PMC article.
-
Problems with evidence assessment in COVID-19 health policy impact evaluation: a systematic review of study design and evidence strength.BMJ Open. 2022 Jan 11;12(1):e053820. doi: 10.1136/bmjopen-2021-053820. BMJ Open. 2022. PMID: 35017250 Free PMC article.
-
Retracted papers on SARS-CoV-2 and COVID-19.Br J Anaesth. 2021 Apr;126(4):e155-e156. doi: 10.1016/j.bja.2021.01.008. Epub 2021 Jan 19. Br J Anaesth. 2021. PMID: 33581852 Free PMC article. No abstract available.
-
From Cytokine Storm to Cytokine Breeze: Did Lessons Learned from Immunopathogenesis Improve Immunomodulatory Treatment of Moderate-to-Severe COVID-19?Biomedicines. 2022 Oct 18;10(10):2620. doi: 10.3390/biomedicines10102620. Biomedicines. 2022. PMID: 36289881 Free PMC article. Review.
References
-
- Hossain MM. Current Status of Global Research on Novel Coronavirus Disease (COVID-19): A Bibliometric Analysis and Knowledge Mapping https://ssrncom/abstract=3547824. 2020.
-
- Greaves S. Sharing findings related to COVID-19 in these extraordinary times www.hindawi.com: Hindawi; [19th April 2020]. https://www.hindawi.com/post/sharing-findings-related-covid-19-these-ext....
-
- PLOS. A message to our community regarding COVID-19 plos.org: PLOS; [updated 19th March, 202019th April, 2020]. https://plos.org/blog/announcement/a-message-to-our-community-regarding-....
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

