Chloroquine and its derivatives in the management of COVID-19: A scoping review
- PMID: 33152192
- PMCID: PMC7676841
- DOI: 10.7705/biomedica.5478
Chloroquine and its derivatives in the management of COVID-19: A scoping review
Abstract
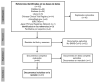
Introduction: Recently, researchers from China and France reported on the effectiveness of chloroquine and hydroxychloroquine for the inhibition of SARS-CoV-2 viral replication in vitro. Timely dissemination of scientific information is key in times of pandemic. A systematic review of the effect and safety of these drugs on COVID-19 is urgently needed. Objective: To map published studies until March 25, 2020, on the use of chloroquine and its derivates in patients with COVID-19. Materials and methods: We searched on PubMed, Embase, Lilacs, and 15 registries from the World Health Organization’s International Clinical Trials Registry Platform for theoretical and empirical research in English, Spanish, Italian, French, or Portuguese until March 25, 2020, and made a narrative synthesis of the results. Results: We included 19 records and 24 trial registries (n=43) including 18,059 patients. China registered 66% (16/24) of the trials. Nine trials evaluate chloroquine exclusively and eight hydroxychloroquine. The records are comments (n=9), in vitro studies (n=3), narrative reviews (n=2), clinical guidelines (n=2), as well as a systematic review, an expert consensus, and a clinical trial. Conclusions: One small (n=26), non-randomized, and flawed clinical trial supports hydroxychloroquine use in patients with COVID-19. There is an urgent need for more clinical trial results to determine the effect and safety of chloroquine and hydroxychloroquine on COVID-19.
Introducción. Recientemente, investigadores chinos y franceses reportaron la eficacia de la cloroquina y la hidroxicloroquina para inhibir la replicación in vitro del virus SARS-CoV-2. La diseminación oportuna de la información científica es clave en tiempos de pandemia. Es urgente contar con una revisión sistemática sobre el efecto y la seguridad de estos medicamentos en la COVID-19. Objetivo. Describir el estado actual de la literatura científica publicada hasta el 25 de marzo de 2020 sobre el uso de la cloroquina o sus derivados en el manejo de pacientes con COVID-19. Materiales y métodos. Se hizo una revisión sistemática exploratoria en PubMed, Embase, Lilacs y 15 bases de datos de la Plataforma de Registros Internacionales de Ensayos Clínicos de la Organización Mundial de la Salud (OMS). Se incluyeron publicaciones empíricas y teóricas en inglés, español, italiano, francés o portugués, y se hizo una síntesis narrativa de los resultados. Resultados. Se incluyeron 19 documentos y 24 registros de ensayos clínicos (n=43) de 18.059 pacientes. El 66 % (16/24) de los ensayos están registrados en China. Nueve ensayos evalúan la cloroquina exclusivamente y ocho, la hidroxicloroquina. Los documentos son comentarios (n=9), estudios in vitro (n=3), revisiones narrativas (n=2), guías de práctica clínica (n=2), así como una revisión sistemática, un consenso de expertos y un ensayo controlado. Conclusiones. Un ensayo clínico pequeño (n=26), no aleatorizado y defectuoso, respalda el uso de la hidroxicloroquina en pacientes con COVID-19. Se requiere de manera urgente tener acceso a los resultados de otros ensayos clínicos para determinar la efectividad y la seguridad de la cloroquina y sus derivados en pacientes con COVID-19.
Keywords: chloroquine; hydroxychloroquine; coronavirus; systematic review; clinical trial; pandemics; SARS-CoV-2.
Similar articles
-
Of chloroquine and COVID-19.Antiviral Res. 2020 May;177:104762. doi: 10.1016/j.antiviral.2020.104762. Epub 2020 Mar 5. Antiviral Res. 2020. PMID: 32147496 Free PMC article. Review.
-
Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19.Eur Rev Med Pharmacol Sci. 2020 Apr;24(8):4539-4547. doi: 10.26355/eurrev_202004_21038. Eur Rev Med Pharmacol Sci. 2020. PMID: 32373993 Review.
-
Safety considerations for chloroquine and hydroxychloroquine in the treatment of COVID-19.Clin Microbiol Infect. 2020 Sep;26(9):1276-1277. doi: 10.1016/j.cmi.2020.05.006. Epub 2020 May 16. Clin Microbiol Infect. 2020. PMID: 32422406 Free PMC article. No abstract available.
-
A systematic review on use of aminoquinolines for the therapeutic management of COVID-19: Efficacy, safety and clinical trials.Life Sci. 2020 Aug 1;254:117775. doi: 10.1016/j.lfs.2020.117775. Epub 2020 May 11. Life Sci. 2020. PMID: 32418894 Free PMC article.
-
Clinical evidence for repurposing chloroquine and hydroxychloroquine as antiviral agents: a systematic review.Clin Microbiol Infect. 2020 Aug;26(8):979-987. doi: 10.1016/j.cmi.2020.05.016. Epub 2020 May 26. Clin Microbiol Infect. 2020. PMID: 32470568 Free PMC article.
Cited by
-
Hydroxychloroquine and Chloroquine in Prophylaxis and Treatment of COVID-19: What Is Known?J Pharm Bioallied Sci. 2021 Jan-Mar;13(1):4-10. doi: 10.4103/jpbs.JPBS_404_20. Epub 2020 Oct 6. J Pharm Bioallied Sci. 2021. PMID: 34084043 Free PMC article. Review.
-
Chloroquine and Hydroxychloroquine Interact Differently with ACE2 Domains Reported to Bind with the Coronavirus Spike Protein: Mediation by ACE2 Polymorphism.Molecules. 2021 Jan 28;26(3):673. doi: 10.3390/molecules26030673. Molecules. 2021. PMID: 33525415 Free PMC article.
References
-
- World Health Organization WHO Director-General's opening remarks at the media briefing on COVID-19. [23 de marzo de 2020]. Disponible en: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re....
-
- World Health Organization Coronavirus disease 2019. [8 de abril de 2020]. Disponible en: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous