Drp1 Tubulates the ER in a GTPase-Independent Manner
- PMID: 33152269
- PMCID: PMC7680448
- DOI: 10.1016/j.molcel.2020.10.013
Drp1 Tubulates the ER in a GTPase-Independent Manner
Abstract
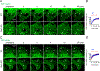
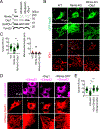
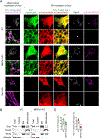
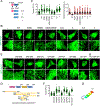
Mitochondria are highly dynamic organelles that continuously grow, divide, and fuse. The division of mitochondria is crucial for human health. During mitochondrial division, the mechano-guanosine triphosphatase (GTPase) dynamin-related protein (Drp1) severs mitochondria at endoplasmic reticulum (ER)-mitochondria contact sites, where peripheral ER tubules interact with mitochondria. Here, we report that Drp1 directly shapes peripheral ER tubules in human and mouse cells. This ER-shaping activity is independent of GTP hydrolysis and located in a highly conserved peptide of 18 amino acids (termed D-octadecapeptide), which is predicted to form an amphipathic α helix. Synthetic D-octadecapeptide tubulates liposomes in vitro and the ER in cells. ER tubules formed by Drp1 promote mitochondrial division by facilitating ER-mitochondria interactions. Thus, Drp1 functions as a two-in-one protein during mitochondrial division, with ER tubulation and mechano-GTPase activities.
Keywords: Drp1; mitochondria; mitochondrial division; organelle contact sites; phosphaditic acid; the endoplasmic reticulum.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



References
-
- Baumann O, and Walz B (2001). Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int Rev Cytol 205, 149–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

