Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): an open-label, multicentre, parallel-group, randomised, phase 3 trial
- PMID: 33152284
- PMCID: PMC7606901
- DOI: 10.1016/S1470-2045(20)30458-7
Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): an open-label, multicentre, parallel-group, randomised, phase 3 trial
Erratum in
-
Correction to Lancet Oncol 2020; 21: 1443-54.Lancet Oncol. 2020 Dec;21(12):e553. doi: 10.1016/S1470-2045(20)30677-X. Lancet Oncol. 2020. PMID: 33271112 Free PMC article. No abstract available.
Abstract
Background: Preoperative and perioperative aromatase inhibitor (POAI) therapy has the potential to improve outcomes in women with operable oestrogen receptor-positive primary breast cancer. It has also been suggested that tumour Ki67 values after 2 weeks (Ki672W) of POAI predicts individual patient outcome better than baseline Ki67 (Ki67B). The POETIC trial aimed to test these two hypotheses.
Methods: POETIC was an open-label, multicentre, parallel-group, randomised, phase 3 trial (done in 130 UK hospitals) in which postmenopausal women aged at least 50 years with WHO performance status 0-1 and hormone receptor-positive, operable breast cancer were randomly assigned (2:1) to POAI (letrozole 2·5 mg per day orally or anastrozole 1 mg per day orally) for 14 days before and following surgery or no POAI (control). Adjuvant treatment was given as per UK standard local practice. Randomisation was done centrally by computer-generated permuted block method (variable block size of six or nine) and was stratified by hospital. Treatment allocation was not masked. The primary endpoint was time to recurrence. A key second objective explored association between Ki67 (dichotomised at 10%) and disease outcomes. The primary analysis for clinical endpoints was by modified intention to treat (excluding patients who withdrew consent). For Ki67 biomarker association and endpoint analysis, the evaluable population included all randomly assigned patients who had paired Ki67 values available. This study is registered with ClinicalTrials.gov, NCT02338310; the European Clinical Trials database, EudraCT2007-003877-21; and the ISRCTN registry, ISRCTN63882543. Recruitment is complete and long-term follow-up is ongoing.
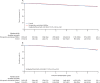
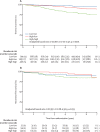
Findings: Between Oct 13, 2008, and April 16, 2014, 4480 women were recruited and randomly assigned to POAI (n=2976) or control (n=1504). On Feb 6, 2018, median follow-up was 62·9 months (IQR 58·1-74·1). 434 (10%) of 4480 women had a breast cancer recurrence (280 [9%] POAI; 154 [10%] control), hazard ratio 0·92 (95% CI 0·75-1·12); p=0·40 with the proportion free from breast cancer recurrence at 5 years of 91·0% (95% CI 89·9-92·0) for patients in the POAI group and 90·4% (88·7-91·9) in the control group. Within the POAI-treated HER2-negative subpopulation, 5-year recurrence risk in women with low Ki67B and Ki672W (low-low) was 4·3% (95% CI 2·9-6·3), 8·4% (6·8-10·5) with high Ki67B and low Ki672W (high-low) and 21·5% (17·1-27·0) with high Ki67B and Ki672W (high-high). Within the POAI-treated HER2-positive subpopulation, 5-year recurrence risk in the low-low group was 10·1% (95% CI 3·2-31·3), 7·7% (3·4-17·5) in the high-low group, and 15·7% (10·1-24·4) in the high-high group. The most commonly reported grade 3 adverse events were hot flushes (20 [1%] of 2801 patients in the POAI group vs six [<1%] of 1400 in the control group) and musculoskeletal pain (29 [1%] vs 13 [1%]). No treatment-related deaths were reported.
Interpretation: POAI has not been shown to improve treatment outcome, but can be used without detriment to help select appropriate adjuvant therapy based on tumour Ki67. Most patients with low Ki67B or low POAI-induced Ki672W do well with adjuvant standard endocrine therapy (giving consideration to clinical-pathological factors), whereas those whose POAI-induced Ki672W remains high might benefit from further adjuvant treatment or trials of new therapies.
Funding: Cancer Research UK.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Implementing preoperative endocrine therapy in breast cancer.Lancet Oncol. 2020 Nov;21(11):1390-1392. doi: 10.1016/S1470-2045(20)30478-2. Lancet Oncol. 2020. PMID: 33152280 No abstract available.
References
-
- Dowsett M, Smith IE, Ebbs SR. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11:951s–958s. - PubMed
-
- Ellis MJ, Coop A, Singh B. Letrozole inhibits tumor proliferation more effectively than tamoxifen independent of HER1/2 expression status. Cancer Res. 2003;63:6523–6531. - PubMed
-
- Fisher B, Gunduz N, Coyle J, Rudock C, Saffer E. Presence of a growth-stimulating factor in serum following primary tumor removal in mice. Cancer Res. 1989;49:1996–2001. - PubMed
-
- Fisher B, Saffer E, Rudock C, Coyle J, Gunduz N. Effect of local or systemic treatment prior to primary tumor removal on the production and response to a serum growth-stimulating factor in mice. Cancer Res. 1989;49:2002–2004. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

