TANK-Binding Kinase 1 Regulates the Localization of Acyl-CoA Synthetase ACSL1 to Control Hepatic Fatty Acid Oxidation
- PMID: 33152322
- PMCID: PMC7710607
- DOI: 10.1016/j.cmet.2020.10.010
TANK-Binding Kinase 1 Regulates the Localization of Acyl-CoA Synthetase ACSL1 to Control Hepatic Fatty Acid Oxidation
Abstract
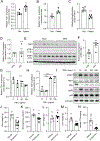
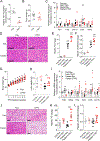
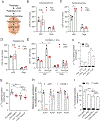
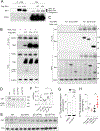
Hepatic TANK (TRAF family member associated NFκB activator)-binding kinase 1 (TBK1) activity is increased during obesity, and administration of a TBK1 inhibitor reduces fatty liver. Surprisingly, liver-specific TBK1 knockout in mice produces fatty liver by reducing fatty acid oxidation. TBK1 functions as a scaffolding protein to localize acyl-CoA synthetase long-chain family member 1 (ACSL1) to mitochondria, which generates acyl-CoAs that are channeled for β-oxidation. TBK1 is induced during fasting and maintained in the unphosphorylated, inactive state, enabling its high affinity binding to ACSL1 in mitochondria. In TBK1-deficient liver, ACSL1 is shifted to the endoplasmic reticulum to promote fatty acid re-esterification in lieu of oxidation in response to fasting, which accelerates hepatic lipid accumulation. The impaired fatty acid oxidation in TBK1-deficient hepatocytes is rescued by the expression of kinase-dead TBK1. Thus, TBK1 operates as a rheostat to direct the fate of fatty acids in hepatocytes, supporting oxidation when inactive during fasting and promoting re-esterification when activated during obesity.
Keywords: acyl-CoA synthetase long-chain family member 1 (ACSL1); fasting; hepatic lipid metabolism; mitochondria; nonalcoholic fatty liver disease (NAFLD); re-esterification; tank-binding kinase 1 (TBK1); β-oxidation.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests A.R.S. is a founder of Elgia Therapeutics and named inventor on several patent applications that include amlexanox. The other authors declare that they have no competing interests.
Figures



References
-
- ANSTEE QM, TARGHER G & DAY CP 2013. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol, 10, 330–44. - PubMed
-
- BLACKBURN P, DESPRÉS JP, LAMARCHE B, TREMBLAY A, BERGERON J, LEMIEUX I & COUILLARD C 2006. Postprandial variations of plasma inflammatory markers in abdominally obese men. Obesity (Silver Spring), 14, 1747–54. - PubMed
-
- BYRNE CD & TARGHER G 2015. NAFLD: a multisystem disease. J Hepatol, 62, S47–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

