Pharmacokinetics of CamSA, a potential prophylactic compound against Clostridioides difficile infections
- PMID: 33152344
- PMCID: PMC7770080
- DOI: 10.1016/j.bcp.2020.114314
Pharmacokinetics of CamSA, a potential prophylactic compound against Clostridioides difficile infections
Abstract
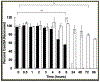
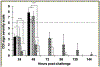
Clostridioides difficile infections (CDI) are the leading cause of nosocomial antibiotic-associated diarrhea. C. difficile produces dormant spores that serve as infectious agents. Bile salts in the gastrointestinal tract signal spores to germinate into toxin-producing cells. As spore germination is required for CDI onset, anti-germination compounds may serve as prophylactics. CamSA, a synthetic bile salt, was previously shown to inhibit C. difficile spore germination in vitro and in vivo. Unexpectedly, a single dose of CamSA was sufficient to offer multi-day protection from CDI in mice without any observable toxicity. To study this intriguing protection pattern, we examined the pharmacokinetic parameters of CamSA. CamSA was stable to the gut of antibiotic-treated mice but was extensively degraded by the microbiota of non-antibiotic-treated animals. Our data also suggest that CamSA's systemic absorption is minimal since it is retained primarily in the intestinal lumen and liver. CamSA shows weak interactions with CYP3A4, a P450 hepatic isozyme involved in drug metabolism and bile salt modification. Like other bile salts, CamSA seems to undergo enterohepatic circulation. We hypothesize that the cycling of CamSA between the liver and intestines serves as a slow-release mechanism that allows CamSA to be retained in the gastrointestinal tract for days. This model explains how a single CamSA dose can prevent murine CDI even though spores are present in the animal's intestine for up to four days post-challenge.
Keywords: Bile salts; CDI; Clostridioides difficile; Drug therapy; Intestine; Liver; Metabolism; Microbiome.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interests
None of the authors have conflict of interests in the publication of this manuscript
Figures

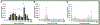
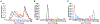

Similar articles
-
An Aniline-Substituted Bile Salt Analog Protects both Mice and Hamsters from Multiple Clostridioides difficile Strains.Antimicrob Agents Chemother. 2022 Jan 18;66(1):e0143521. doi: 10.1128/AAC.01435-21. Epub 2021 Nov 15. Antimicrob Agents Chemother. 2022. PMID: 34780262 Free PMC article.
-
Fate of ingested Clostridium difficile spores in mice.PLoS One. 2013 Aug 30;8(8):e72620. doi: 10.1371/journal.pone.0072620. eCollection 2013. PLoS One. 2013. PMID: 24023628 Free PMC article.
-
A new strategy for the prevention of Clostridium difficile infection.J Infect Dis. 2013 May 15;207(10):1498-504. doi: 10.1093/infdis/jit068. Epub 2013 Feb 18. J Infect Dis. 2013. PMID: 23420906
-
Bile acids as germinants for Clostridioides difficile spores, evidence of adaptation to the gut?FEMS Microbiol Rev. 2025 Jan 14;49:fuaf005. doi: 10.1093/femsre/fuaf005. FEMS Microbiol Rev. 2025. PMID: 39924167 Free PMC article. Review.
-
Inhibition of spores to prevent the recurrence of Clostridioides difficile infection - A possibility or an improbability?J Microbiol Immunol Infect. 2021 Dec;54(6):1011-1017. doi: 10.1016/j.jmii.2021.06.002. Epub 2021 Jun 26. J Microbiol Immunol Infect. 2021. PMID: 34229970 Review.
Cited by
-
Phenotypic analysis of various Clostridioides difficile ribotypes reveals consistency among core processes.bioRxiv [Preprint]. 2025 Jan 10:2025.01.10.632434. doi: 10.1101/2025.01.10.632434. bioRxiv. 2025. Update in: Appl Environ Microbiol. 2025 Jul 23;91(7):e0096425. doi: 10.1128/aem.00964-25. PMID: 39829883 Free PMC article. Updated. Preprint.
-
An Aniline-Substituted Bile Salt Analog Protects both Mice and Hamsters from Multiple Clostridioides difficile Strains.Antimicrob Agents Chemother. 2022 Jan 18;66(1):e0143521. doi: 10.1128/AAC.01435-21. Epub 2021 Nov 15. Antimicrob Agents Chemother. 2022. PMID: 34780262 Free PMC article.
-
Efficacy of Omadacycline or Vancomycin Combined With Germinants for Preventing Clostridioides difficile Relapse in a Murine Model.J Infect Dis. 2023 Mar 1;227(5):622-630. doi: 10.1093/infdis/jiac324. J Infect Dis. 2023. PMID: 35904942 Free PMC article.
-
Bile acid-independent protection against Clostridioides difficile infection.PLoS Pathog. 2021 Oct 19;17(10):e1010015. doi: 10.1371/journal.ppat.1010015. eCollection 2021 Oct. PLoS Pathog. 2021. PMID: 34665847 Free PMC article.
-
Clostridioides difficile spore germination: initiation to DPA release.Curr Opin Microbiol. 2022 Feb;65:101-107. doi: 10.1016/j.mib.2021.11.001. Epub 2021 Nov 19. Curr Opin Microbiol. 2022. PMID: 34808546 Free PMC article. Review.
References
-
- Yip C, Phan JR & Abel-Santos E Treatment of Clostridium difficile Infections. RSC Drug Discovery Series vol. January (2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

