BRAF Mutation in Colorectal Cancers: From Prognostic Marker to Targetable Mutation
- PMID: 33152998
- PMCID: PMC7694028
- DOI: 10.3390/cancers12113236
BRAF Mutation in Colorectal Cancers: From Prognostic Marker to Targetable Mutation
Abstract
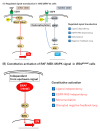
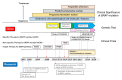
The Raf murine sarcoma viral oncogene homolog B (BRAF) mutation is detected in 8-12% of metastatic colorectal cancers (mCRCs) and is strongly correlated with poor prognosis. The recent success of the BEACON CRC study and the development of targeted therapy have led to the determination of BRAF-mutated mCRCs as an independent category. For nearly two decades, a growing body of evidence has established the significance of the BRAF mutation in the development of CRC. Herein, we overview both basic and clinical data relevant to BRAF-mutated CRC, mainly focusing on the development of treatment strategies. This review is organized into eight sections, including clinicopathological features, molecular features, prognosis, the predictive value of anti-epidermal growth factor receptor (EGFR) therapy, resistant mechanisms for BRAF-targeting treatment, the heterogeneity of the BRAF mutation, future perspectives, and conclusions. A characterization of the canonical mitogen-activated protein kinase (MAPK) pathway is essential for controlling this malignancy, and the optimal combination of multiple interventions for treatments remains a point of debate.
Keywords: BRAF inhibitor; RAF–MEK–ERK signaling pathway; cancer precision medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Li W.Q., Kawakami K., Ruszkiewicz A., Bennett G., Moore J., Iacopetta B. BRAF mutations are associated with distinctive clinical, pathological and molecular features of colorectal cancer independently of microsatellite instability status. Mol. Cancer. 2006;5:2. doi: 10.1186/1476-4598-5-2. - DOI - PMC - PubMed
-
- Seligmann J.F., Fisher D., Smith C.G., Richman S.D., Elliott F., Brown S., Adams R., Maughan T., Quirke P., Cheadle J., et al. Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: Analysis from 2530 patients in randomized clinical trials. Ann. Oncol. 2017;28:562–568. doi: 10.1093/annonc/mdw645. - DOI - PubMed
-
- Van Cutsem E., Huijberts S., Grothey A., Yaeger R., Cuyle P.J., Elez E., Fakih M., Montagut C., Peeters M., Yoshino T., et al. Binimetinib, Encorafenib, and Cetuximab Triplet Therapy for Patients with BRAF V600E-Mutant Metastatic Colorectal Cancer: Safety Lead-In Results from the Phase III BEACON Colorectal Cancer Study. J. Clin. Oncol. 2019;37:1460–1469. doi: 10.1200/JCO.18.02459. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

