Glutamatergic Receptor Trafficking and Delivery: Role of the Exocyst Complex
- PMID: 33153008
- PMCID: PMC7693776
- DOI: 10.3390/cells9112402
Glutamatergic Receptor Trafficking and Delivery: Role of the Exocyst Complex
Abstract
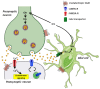
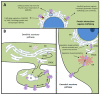
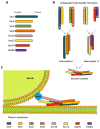
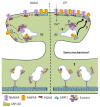
Cells comprise several intracellular membrane compartments that allow them to function properly. One of these functions is cargo movement, typically proteins and membranes within cells. These cargoes ride microtubules through vesicles from Golgi and recycling endosomes to the plasma membrane in order to be delivered and exocytosed. In neurons, synaptic functions employ this cargo trafficking to maintain inter-neuronal communication optimally. One of the complexes that oversee vesicle trafficking and tethering is the exocyst. The exocyst is a protein complex containing eight subunits first identified in yeast and then characterized in multicellular organisms. This complex is related to several cellular processes, including cellular growth, division, migration, and morphogenesis, among others. It has been associated with glutamatergic receptor trafficking and tethering into the synapse, providing the molecular machinery to deliver receptor-containing vesicles into the plasma membrane in a constitutive manner. In this review, we discuss the evidence so far published regarding receptor trafficking and the exocyst complex in both basal and stimulated levels, comparing constitutive trafficking and long-term potentiation-related trafficking.
Keywords: LTP-induced trafficking; constitutive trafficking; exocyst; glutamatergic receptor; membrane trafficking; synapse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

