Safety and Immunogenicity of the GamTBvac, the Recombinant Subunit Tuberculosis Vaccine Candidate: A Phase II, Multi-Center, Double-Blind, Randomized, Placebo-Controlled Study
- PMID: 33153191
- PMCID: PMC7712213
- DOI: 10.3390/vaccines8040652
Safety and Immunogenicity of the GamTBvac, the Recombinant Subunit Tuberculosis Vaccine Candidate: A Phase II, Multi-Center, Double-Blind, Randomized, Placebo-Controlled Study
Abstract
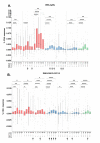
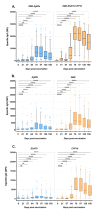
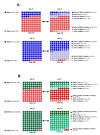
GamTBvac is a candidate tuberculosis vaccine with two fusion proteins, containing Ag85a, ESAT6, CFP10, and a dextran-binding domain (DBD). Phase II of a double-blind, randomized, multicenter, placebo-controlled study in parallel groups in healthy adults to evaluate the safety and immunogenicity of GamTBvac in 180 previously-vaccinated with Bacillus Calmette-Guérin vaccine (BCG) healthy volunteers without Mycobacterium tuberculosis (MTB) infection was conducted. The dose (0.5 mL) of either the study drug or a placebo was administered subcutaneously twice with an 8-week interval. At eight timepoints from 14 to 150 days, whole blood and sera were assayed. Antigen-specific T-cell responses were measured by an in-house interferon-gamma release assay (IGRA-test), the QuantiFERON (QTF) test, and intracellular cytokine staining (ICS). For antibody response detection, the bead-based multiplex immunoassay (MIA) was applied. The vaccine confirmed an acceptable safety profile previously shown in a first-in-human clinical study. After stimulation with both fusions, the highest median level of INF-γ was detected on day 21. The GamTBvac vaccine induced antigen-specific interferon-gamma release, Th1 cytokine-expressing CD4+ T-cells, and IgG responses and results support further clinical testing of GamTBvac.
Keywords: BCG booster; clinical trials; safety and immunogenicity; subunit vaccine; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of this study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- WHO|Global Tuberculosis Report. [(accessed on 22 June 2020)];2019 Available online: http://www.who.int/tb/publications/global_report/en/
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

