Sestrin2 regulates microglia polarization through mTOR-mediated autophagic flux to attenuate inflammation during experimental brain ischemia
- PMID: 33153476
- PMCID: PMC7643276
- DOI: 10.1186/s12974-020-01987-y
Sestrin2 regulates microglia polarization through mTOR-mediated autophagic flux to attenuate inflammation during experimental brain ischemia
Abstract
Background: Neuroinflammation is the major pathogenesis of cerebral ischemia. Microglia are activated and polarized to either the pro-inflammatory M1 phenotype or anti-inflammatory M2 phenotype, which act as a critical mediator of neuroinflammation. Sestrin2 has pro-survival properties against ischemic brain injury. However, whether sestrin2 has an anti-inflammatory function by shifting microglia polarization and its underlying mechanism is unknown.
Methods: Adult male C57BL/6 mice (N = 108) underwent transient middle cerebral artery occlusion (tMCAO) and were treated with exogenous sestrin2. Neurological deficit scores and infarct volume were determined. Cell apoptosis was examined by TUNEL staining and Western blotting. The expression of inflammatory mediators, M1/M2-specific markers, and signaling pathways were detected by reverse transcription-polymerase chain reaction, immunostaining, and Western blotting. To explore the underlying mechanism, primary neurons were subjected to oxygen-glucose deprivation (OGD) and then treated with oxygenated condition medium of BV2 cells incubated with different doses of sestrin2.
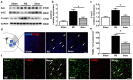
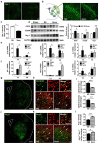
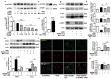
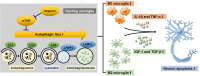
Results: Sestrin2 attenuated the neurological deficits, infarction volume, and cell apoptosis after tMCAO compared to those in the control (p < 0.05). Sestrin2 had an anti-inflammatory effect and could suppress M1 microglia polarization and promote M2 microglia polarization. Condition medium from BV2 cells cultured with sestrin2 reduced neuronal apoptosis after OGD in vitro. Furthermore, we demonstrated that sestrin2 drives microglia to the M2 phenotype by inhibiting the mammalian target of rapamycin (mTOR) signaling pathway and restoring autophagic flux.
Conclusions: Sestrin2 exhibited neuroprotection by shifting microglia polarization from the M1 to M2 phenotype in ischemic mouse brain, which may be due to suppression of the mTOR signaling pathway and the restoration of autophagic flux.
Keywords: Autophagic flux; Inflammation; Ischemia; Microglia; mTOR.
Conflict of interest statement
The authors declare no potential conflicts of interest in relation to this study.
Figures


References
MeSH terms
Substances
Grants and funding
- 2019-01-07-00-02-E00064/Scientific Research and Innovation Program of Shanghai Education Commission
- 2016YFC1300600/National Key Research and Development Program of China
- 81771251/National Natural Science Foundation of China
- 81771251/National Natural Science Foundation of China
- 81974179/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Miscellaneous

