TM4SF1 promotes EMT and cancer stemness via the Wnt/β-catenin/SOX2 pathway in colorectal cancer
- PMID: 33153498
- PMCID: PMC7643364
- DOI: 10.1186/s13046-020-01690-z
TM4SF1 promotes EMT and cancer stemness via the Wnt/β-catenin/SOX2 pathway in colorectal cancer
Abstract
Background: Transmembrane 4 L six family member 1 (TM4SF1) is upregulated in several epithelial cancers and is closely associated with poor prognosis. However, the role of TM4SF1 and its potential mechanism in colorectal cancer (CRC) remain elusive.
Methods: We investigated the expression of TM4SF1 in the Oncomine, the Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) databases and confirmed the results by immunohistochemistry (IHC), qPCR and Western blotting (WB) of CRC tissues. The effect of TM4SF1 on the epithelial-to-mesenchymal transition (EMT) and cancer stemness of CRC cells was investigated by Transwell, wound healing and sphere formation assays. A series of in vitro and in vivo experiments were conducted to reveal the mechanisms by which TM4SF1 modulates EMT and cancer stemness in CRC.
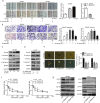
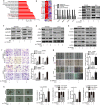
Results: TM4SF1 expression was markedly higher in CRC tissues than in non-tumour tissues and was positively correlated with poor prognosis. Downregulation of TM4SF1 inhibited the migration, invasion and tumour sphere formation of SW480 and LoVo cells. Conversely, TM4SF1 overexpression significantly enhanced the migration, invasion and tumoursphere formation potential of CRC cells, Additionally, TM4SF1 silencing inhibited the EMT mediated by transforming growth factor-β1 (TGF-β1). Mechanistically, gene set enrichment analysis (GSEA) predicted that the Wnt signalling pathway was one of the most impaired pathways in TM4SF1-deficient CRC cells compared to controls. The results were further validated by WB, which revealed that TM4SF1 modulated SOX2 expression in a Wnt/β-catenin activation-dependent manner. Furthermore, we found that knockdown of TM4SF1 suppressed the expression of c-Myc, leading to decreased c-Myc binding to the SOX2 gene promoter. Finally, depletion of TM4SF1 inhibited metastasis and tumour growth in a xenograft mouse model.
Conclusion: Our study substantiates a novel mechanism by which TM4SF1 maintains cancer cell stemness and EMT via the Wnt/β-catenin/c-Myc/SOX2 axis during the recurrence and metastasis of CRC.
Keywords: Colorectal cancer; EMT; SOX2; Stemness; TM4SF1; Wnt/β-catenin.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Hellstrom IHD, Linsley P, Brown JP, Brankovan V, Hellstrom KE. Monoclonal mouse antibodies raised against human lung carcinoma. Elsevier. 1987;3(1):3917–23. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

