Anticoagulation in COVID-19: A Systematic Review, Meta-analysis, and Rapid Guidance From Mayo Clinic
- PMID: 33153635
- PMCID: PMC7458092
- DOI: 10.1016/j.mayocp.2020.08.030
Anticoagulation in COVID-19: A Systematic Review, Meta-analysis, and Rapid Guidance From Mayo Clinic
Abstract
A higher risk of thrombosis has been described as a prominent feature of coronavirus disease 2019 (COVID-19). This systematic review synthesizes current data on thrombosis risk, prognostic implications, and anticoagulation effects in COVID-19. We included 37 studies from 4070 unique citations. Meta-analysis was performed when feasible. Coagulopathy and thrombotic events were frequent among patients with COVID-19 and further increased in those with more severe forms of the disease. We also present guidance on the prevention and management of thrombosis from a multidisciplinary panel of specialists from Mayo Clinic. The current certainty of evidence is generally very low and continues to evolve.
Copyright © 2020 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc. All rights reserved.
Figures
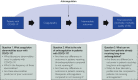
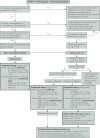
References
-
- Johns Hopkins University COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. https://coronavirus.jhu.edu/map.html
-
- Centers for Disease Control and Prevention The Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET) https://gis.cdc.gov/grasp/covidnet/COVID19_3.html
-
- Rota P.A., Oberste M.S., Monroe S.S. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300(5624):1394–1399. - PubMed
-
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia [published correction appears in N Engl J Med. 2013;369(4):394] N Engl J Med. 2012;367(19):1814–1820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

