Therapeutic Ultrasound Parameter Optimization for Drug Delivery Applied to a Murine Model of Hepatocellular Carcinoma
- PMID: 33153807
- PMCID: PMC8489309
- DOI: 10.1016/j.ultrasmedbio.2020.09.009
Therapeutic Ultrasound Parameter Optimization for Drug Delivery Applied to a Murine Model of Hepatocellular Carcinoma
Abstract
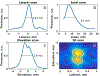
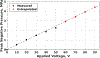
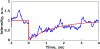
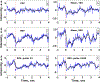
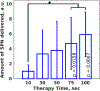
Ultrasound and microbubble (USMB)-mediated drug delivery is a valuable tool for increasing the efficiency of the delivery of therapeutic agents to cancer while maintaining low systemic toxicity. Typically, selection of USMB drug delivery parameters used in current research settings are either based on previous studies described in the literature or optimized using tissue-mimicking phantoms. However, phantoms rarely mimic in vivo tumor environments, and the selection of parameters should be based on the application or experiment. In the following study, we optimized the therapeutic parameters of the ultrasound drug delivery system to achieve the most efficient in vivo drug delivery using fluorescent semiconducting polymer nanoparticles as a model nanocarrier. We illustrate that voltage, pulse repetition frequency and treatment time (i.e., number of ultrasound pulses per therapy area) delivered to the tumor can successfully be optimized in vivo to ensure effective delivery of the semiconducting polymer nanoparticles to models of hepatocellular carcinoma. The optimal in vivo parameters for USMB drug delivery in this study were 70 V (peak negative pressure = 3.4 MPa, mechanical index = 1.22), 1-Hz pulse repetition frequency and 100-s therapy time. USMB-mediated drug delivery using in vivo optimized ultrasound parameters caused an up to 2.2-fold (p < 0.01) increase in drug delivery to solid tumors compared with that using phantom-optimized ultrasound parameters.
Keywords: Drug delivery; Hepatocellular carcinoma; Microbubbles; Sonoporation; Therapy; Ultrasound.
Copyright © 2020 World Federation for Ultrasound in Medicine & Biology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest disclosure The authors declare no competing interests.
Figures






References
-
- Averkiou M, Powers J, Skyba D, Bruce M, Jensen S, 2003. Ultrasound contrast imaging research. Ultrasound Q. 19 (1), 27–37. - PubMed
-
- Bao L, Yan Y, Xu C, Ji W, Shen S, Xu G, Zeng Y, Sun B, Qian H, Chen L, Wu M, Su C, Chen J, 2013. MicroRNA-21 suppresses PTEN and hSulf-1 expression and promotes hepatocellular carcinoma progression through AKT/ERK pathor2. Cancer Lett. 337 (2), 226–236. - PubMed
-
- Bao S, Thrall BD, Miller DL, 1997. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med. Biol. 23 (6), 953–959. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

