Regulation of Intestinal UDP-Glucuronosyltransferase 1A1 by the Farnesoid X Receptor Agonist Obeticholic Acid Is Controlled by Constitutive Androstane Receptor through Intestinal Maturation
- PMID: 33154041
- PMCID: PMC7745083
- DOI: 10.1124/dmd.120.000240
Regulation of Intestinal UDP-Glucuronosyltransferase 1A1 by the Farnesoid X Receptor Agonist Obeticholic Acid Is Controlled by Constitutive Androstane Receptor through Intestinal Maturation
Abstract
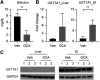
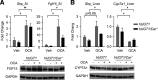
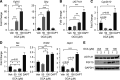
UDP-glucuronosyltransferase (UGT) 1A1 is the only transferase capable of conjugating serum bilirubin. However, temporal delay in the development of the UGT1A1 gene leads to an accumulation of serum bilirubin in newborn children. Neonatal humanized UGT1 (hUGT1) mice, which accumulate severe levels of total serum bilirubin (TSB), were treated by oral gavage with obeticholic acid (OCA), a potent FXR agonist. OCA treatment led to dramatic reduction in TSB levels. Analysis of UGT1A1 expression confirmed that OCA induced intestinal and not hepatic UGT1A1. Interestingly, Cyp2b10, a target gene of the nuclear receptor CAR, was also induced by OCA in intestinal tissue. In neonatal hUGT1/Car -/- mice, OCA was unable to induce CYP2B10 and UGT1A1, confirming that CAR and not FXR is involved in the induction of intestinal UGT1A1. However, OCA did induce FXR target genes, such as Shp, in both intestines and liver with induction of Fgf15 in intestinal tissue. Circulating FGF15 activates hepatic FXR and, together with hepatic Shp, blocks Cyp7a1 and Cyp7b1 gene expression, key enzymes in bile acid metabolism. Importantly, the administration of OCA in neonatal hUGT1 mice accelerates intestinal epithelial cell maturation, which directly impacts on induction of the UGT1A1 gene and the reduction in TSB levels. Accelerated intestinal maturation is directly controlled by CAR, since induction of enterocyte marker genes sucrase-isomaltase, alkaline phosphatase 3, and keratin 20 by OCA does not occur in hUGT1/Car -/- mice. Thus, new findings link an important role for CAR in intestinal UGT1A1 induction and its role in the intestinal maturation pathway. SIGNIFICANCE STATEMENT: Obeticholic acid (OCA) activates FXR target genes in both liver and intestinal tissues while inducing intestinal UGT1A1, which leads to the elimination of serum bilirubin in humanized UGT1 mice. However, the induction of intestinal UGT1A1 and the elimination of bilirubin by OCA is driven entirely by activation of intestinal CAR and not FXR. The elimination of serum bilirubin is based on a CAR-dependent mechanism that facilitates the acceleration of intestinal epithelium cell differentiation, an event that underlies the induction of intestinal UGT1A1.
Copyright © 2020 by The American Society for Pharmacology and Experimental Therapeutics.
Figures




References
-
- Bhutani VK, Johnson-Hamerman L. (2015) The clinical syndrome of bilirubin-induced neurologic dysfunction. Semin Fetal Neonatal Med 20:6–13. - PubMed
-
- Bhutani VK, Zipursky A, Blencowe H, Khanna R, Sgro M, Ebbesen F, Bell J, Mori R, Slusher TM, Fahmy N, et al. (2013) Neonatal hyperbilirubinemia and Rhesus disease of the newborn: incidence and impairment estimates for 2010 at regional and global levels. Pediatr Res 74 (Suppl 1):86–100. - PMC - PubMed
-
- Chen S, Lu W, Yueh MF, Rettenmeier E, Liu M, Paszek M, Auwerx J, Yu RT, Evans RM, Wang K, et al. (2017) Intestinal NCoR1, a regulator of epithelial cell maturation, controls neonatal hyperbilirubinemia [published correction appears in Proc Natl Acad Sci USA (2017) 114:E4115]. Proc Natl Acad Sci USA 114:E1432–E1440. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

