Quality evaluation of case series describing four-factor prothrombin complex concentrate in oral factor Xa inhibitor-associated bleeding: a systematic review
- PMID: 33154059
- PMCID: PMC7646359
- DOI: 10.1136/bmjopen-2020-040499
Quality evaluation of case series describing four-factor prothrombin complex concentrate in oral factor Xa inhibitor-associated bleeding: a systematic review
Abstract
Introduction: As oral factor Xa (oFXa) inhibitor use has increased, so has publication of case series describing related bleeding managed with four-factor prothrombin complex concentrate (4F-PCC).
Objective: This review aimed to identify case series describing 4F-PCC management of oFXa inhibitor-related bleeding and appraise their methodological and reporting quality.
Design: We searched Medline and EMBASE (1 January 2011 to 31 May 2020) to identify series of ≥10 patients with oFXa inhibitor-related major bleeding given off-label 4F-PCC. Case series were evaluated using a validated tool adapted for this topic. The tool addressed patient selection, bleed/outcome ascertainment, causal/temporal association and reporting.
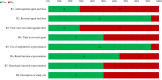
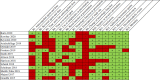
Results: We identified 14 case series. None had ≥100 patients (range=13-84), three were prospective, two detailed appropriate inclusion criteria and four noted consecutive inclusion. While 12 series provided clear/appropriate methods for diagnosis of intracranial haemorrhage (ICH); none did so for extracranial bleeds and it was not clear whether bleeding was adjudicated in any. Haemostatic effectiveness, thrombosis and mortality were together evaluated in 12 series, but only seven used validated methods to evaluate/diagnosis haemostasis in ICH, six in gastrointestinal bleeds, five in other bleeds and three in thrombosis. Independent adjudication of haemostasis (n=1) and thrombosis (n=2) was infrequent. Thirty-day follow-up for mortality and thrombosis was noted in five and seven series. Anticoagulation measurement/levels in at least some patients were conveyed in three series. Few series provided data on anticoagulant agent/dose (n=4), time from anticoagulant (n=4), time-to-reversal (n=7), baseline (n=7) or change (n=0) in neurologic function.
Conclusions: Although many case series describe off-label use of 4F-PCC for oFXa inhibitor-related bleeding, methodological flaws and/or poor reporting necessitates caution in interpretation.
Keywords: anticoagulation; cardiology; haematology; neurology.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: OSC, YR-M and MW have no competing interest to disclose. BL and KM-P are employees of Portola Pharmaceuticals. WB has received consultancy fees from Bayer. CIC has received grant funding and consultancy fees from Janssen Scientific Affairs and Bayer.
Figures








Similar articles
-
Utilization of 4-Factor Prothrombin Complex Concentrate for Reversal of Oral Factor Xa Inhibitor-Associated Acute Major Bleeding: A Case Series.J Pharm Pract. 2021 Oct;34(5):755-760. doi: 10.1177/0897190020907012. Epub 2020 Feb 24. J Pharm Pract. 2021. PMID: 32089040
-
Andexanet alfa versus four-factor prothrombin complex concentrate for the reversal of apixaban- or rivaroxaban-associated intracranial hemorrhage: a propensity score-overlap weighted analysis.Crit Care. 2022 Jun 16;26(1):180. doi: 10.1186/s13054-022-04043-8. Crit Care. 2022. PMID: 35710578 Free PMC article.
-
Safety, efficacy, and cost of four-factor prothrombin complex concentrate (4F-PCC) in patients with factor Xa inhibitor-related bleeding: a retrospective study.J Thromb Thrombolysis. 2019 Aug;48(2):250-255. doi: 10.1007/s11239-019-01846-5. J Thromb Thrombolysis. 2019. PMID: 30941571
-
Effect of low- versus high-dose 4-factor prothrombin complex concentrate in factor Xa inhibitor-associated bleeding: A qualitative systematic review.Am J Health Syst Pharm. 2024 May 24;81(11):e274-e282. doi: 10.1093/ajhp/zxae009. Am J Health Syst Pharm. 2024. PMID: 38430127
-
Management of direct factor Xa inhibitor-related major bleeding with prothrombin complex concentrate: a meta-analysis.Blood Adv. 2019 Jan 22;3(2):158-167. doi: 10.1182/bloodadvances.2018024133. Blood Adv. 2019. PMID: 30658963 Free PMC article. Review.
Cited by
-
Management of Coagulopathy in Bleeding Patients.J Clin Med. 2021 Dec 21;11(1):1. doi: 10.3390/jcm11010001. J Clin Med. 2021. PMID: 35011742 Free PMC article. Review.
-
American College of Gastroenterology-Canadian Association of Gastroenterology Clinical Practice Guideline: Management of Anticoagulants and Antiplatelets During Acute Gastrointestinal Bleeding and the Periendoscopic Period.J Can Assoc Gastroenterol. 2022 Mar 17;5(2):100-101. doi: 10.1093/jcag/gwac010. eCollection 2022 Apr. J Can Assoc Gastroenterol. 2022. PMID: 35368325 Free PMC article. Review.
-
3-Factor prothrombin complex concentrate versus 4-factor prothrombin complex concentrate for the reversal of oral factor Xa inhibitors.J Thromb Thrombolysis. 2025 Feb;58(2):276-283. doi: 10.1007/s11239-024-03052-4. Epub 2024 Oct 28. J Thromb Thrombolysis. 2025. PMID: 39467897 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
