4-Phenylbutyric acid enhances the mineralization of osteogenesis imperfecta iPSC-derived osteoblasts
- PMID: 33154166
- PMCID: PMC7948972
- DOI: 10.1074/jbc.RA120.014709
4-Phenylbutyric acid enhances the mineralization of osteogenesis imperfecta iPSC-derived osteoblasts
Abstract
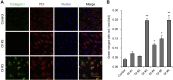
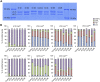
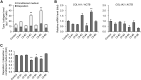
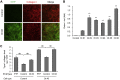
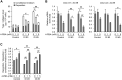
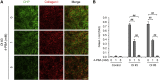
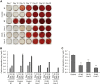
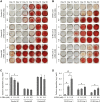
Osteogenesis imperfecta (OI) is a heritable brittle bone disease mainly caused by mutations in the two type I collagen genes. Collagen synthesis is a complex process including trimer formation, glycosylation, secretion, extracellular matrix (ECM) formation, and mineralization. Using OI patient-derived fibroblasts and induced pluripotent stem cells (iPSCs), we investigated the effect of 4-phenylbutyric acid (4-PBA) on collagen synthesis to test its potential as a new treatment for OI. Endoplasmic reticulum (ER) retention of type I collagen was observed by immunofluorescence staining in OI patient-derived fibroblasts with glycine substitution and exon skipping mutations. Liquid chromatography-mass spectrometry analysis revealed excessive glycosylation of secreted type I collagen at the specific sites in OI cells. The misfolding of the type I collagen triple helix in the ECM was demonstrated by the incorporation of heat-dissociated collagen hybridizing peptide in OI cells. Type I collagen was produced excessively by OI fibroblasts with a glycine mutation, but this excessive production was normalized when OI fibroblasts were cultured on control fibroblast-derived ECM. We also found that mineralization was impaired in osteoblasts differentiated from OI iPSCs. In summary, treatment with 4-PBA normalizes the excessive production of type I collagen, reduces ER retention, partially improves misfolding of the type I collagen helix in ECM, and improves osteoblast mineralization. Thus, 4-PBA may improve not only ER retention, but also type I collagen synthesis and mineralization in human cells from OI patients.
Keywords: endoplasmic reticulum (ER); extracellular matrix; fibril; glycosylation; osteoblast.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Marini J.C., Forlino A., Cabral W.A., Barnes A.M., San Antonio J.D., Milgrom S., Hyland J.C., Korkko J., Prockop D.J., De Paepe A., Coucke P., Symoens S., Glorieux F.H., Roughley P.J., Lund A.M. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum. Mutat. 2007;28:209–221. - PMC - PubMed
-
- Ohata Y., Takeyari S., Nakano Y., Kitaoka T., Nakayama H., Bizaoui V., Yamamoto K., Miyata K., Yamamoto K., Fujiwara M., Kubota T., Michigami T., Yamamoto K., Yamamoto T., Namba N. Comprehensive genetic analyses using targeted next-generation sequencing and genotype-phenotype correlations in 53 Japanese patients with osteogenesis imperfecta. Osteoporos. Int. 2019;30:2333–2342. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

