Stem cell fate determination through protein O-GlcNAcylation
- PMID: 33154167
- PMCID: PMC7948975
- DOI: 10.1074/jbc.REV120.014915
Stem cell fate determination through protein O-GlcNAcylation
Abstract
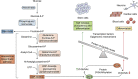
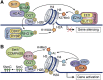
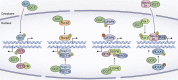
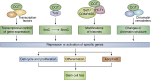
Embryonic and adult stem cells possess the capability of self-renewal and lineage-specific differentiation. The intricate balance between self-renewal and differentiation is governed by developmental signals and cell-type-specific gene regulatory mechanisms. A perturbed intra/extracellular environment during lineage specification could affect stem cell fate decisions resulting in pathology. Growing evidence demonstrates that metabolic pathways govern epigenetic regulation of gene expression during stem cell fate commitment through the utilization of metabolic intermediates or end products of metabolic pathways as substrates for enzymatic histone/DNA modifications. UDP-GlcNAc is one such metabolite that acts as a substrate for enzymatic mono-glycosylation of various nuclear, cytosolic, and mitochondrial proteins on serine/threonine amino acid residues, a process termed protein O-GlcNAcylation. The levels of GlcNAc inside the cells depend on the nutrient availability, especially glucose. Thus, this metabolic sensor could modulate gene expression through O-GlcNAc modification of histones or other proteins in response to metabolic fluctuations. Herein, we review evidence demonstrating how stem cells couple metabolic inputs to gene regulatory pathways through O-GlcNAc-mediated epigenetic/transcriptional regulatory mechanisms to govern self-renewal and lineage-specific differentiation programs. This review will serve as a primer for researchers seeking to better understand how O-GlcNAc influences stemness and may catalyze the discovery of new stem-cell-based therapeutic approaches.
Keywords: O-GlcNAcylation; cell fate determination; epigenetics; gene expression; transcription.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures
References
-
- Conaghan J., Handyside A.H., Winston R.M., Leese H.J. Effects of pyruvate and glucose on the development of human preimplantation embryos in vitro. J. Reprod. Fertil. 1993;99:87–95. - PubMed
-
- Brinster R.L. Embryo development. J. Anim. Sci. 1974;38:1003–1012. - PubMed
-
- Heilig C., Brosius F., Siu B., Concepcion L., Mortensen R., Heilig K., Zhu M., Weldon R., Wu G., Conner D. Implications of glucose transporter protein type 1 (GLUT1)-haplodeficiency in embryonic stem cells for their survival in response to hypoxic stress. Am. J. Pathol. 2003;163:1873–1885. - PMC - PubMed
-
- Miyazawa H., Aulehla A. Revisiting the role of metabolism during development. Development. 2018;145 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

