A universal dual mechanism immunotherapy for the treatment of influenza virus infections
- PMID: 33154358
- PMCID: PMC7645797
- DOI: 10.1038/s41467-020-19386-5
A universal dual mechanism immunotherapy for the treatment of influenza virus infections
Abstract
Seasonal influenza epidemics lead to 3-5 million severe infections and 290,000-650,000 annual global deaths. With deaths from the 1918 influenza pandemic estimated at >50,000,000 and future pandemics anticipated, the need for a potent influenza treatment is critical. In this study, we design and synthesize a bifunctional small molecule by conjugating the neuraminidase inhibitor, zanamivir, with the highly immunogenic hapten, dinitrophenyl (DNP), which specifically targets the surface of free virus and viral-infected cells. We show that this leads to simultaneous inhibition of virus release, and immune-mediated elimination of both free virus and virus-infected cells. Intranasal or intraperitoneal administration of a single dose of drug to mice infected with 100x MLD50 virus is shown to eradicate advanced infections from representative strains of both influenza A and B viruses. Since treatments of severe infections remain effective up to three days post lethal inoculation, our approach may successfully treat infections refractory to current therapies.
Conflict of interest statement
Xin Liu and Philip S. Low have applied through Purdue University for a provisional patent that covers the therapy described in this paper. Other authors declare no competing interests.
Figures


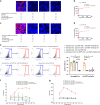

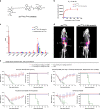

References
-
- Centers for Disease Control and Prevention (CDC), Estimated Range of Annual Burden of Flu in the U.S. since 2010; www.cdc.gov/flu/about/burden.
-
- Centers for Disease Control and Prevention (CDC), Influenza (Flu) in the Workplace; www.cdc.gov/niosh/topics/flu/activities.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

