The smart activatable P2&3TT probe allows accurate, fast, and highly sensitive detection of Staphylococcus aureus in clinical blood culture samples
- PMID: 33154413
- PMCID: PMC7645595
- DOI: 10.1038/s41598-020-76254-4
The smart activatable P2&3TT probe allows accurate, fast, and highly sensitive detection of Staphylococcus aureus in clinical blood culture samples
Abstract
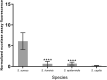
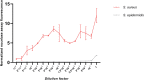
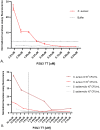
Staphylococcus aureus bacteraemia (SAB) is associated with high mortality and morbidity rates. Yet, there is currently no adequate diagnostic test for early and rapid diagnosis of SAB. Therefore, this study was aimed at exploring the potential for clinical implementation of a nuclease-activatable fluorescent probe for early diagnosis of SAB. To this end, clinical blood culture samples from patients with bloodstream infections were incubated for 1 h with the "smart" activatable P2&3TT probe, the total assay time being less than 2 h. Cleavage of this probe by the secreted S. aureus enzyme micrococcal nuclease results in emission of a readily detectable fluorescence signal. Incubation of S. aureus-positive blood culture samples with the P2&3TT probe resulted in 50-fold higher fluorescence intensity levels than incubation with culture-negative samples. Moreover, incubation of the probe with non-S. aureus-positive blood cultures yielded essentially background fluorescence intensity levels for cultures with Gram-negative bacteria, and only ~ 3.5-fold increased fluorescence intensity levels over background for cultures with non-S. aureus Gram-positive bacteria. Importantly, the measured fluorescence intensities were dose-dependent, and a positive signal was clearly detectable for S. aureus-positive blood cultures with bacterial loads as low as ~ 7,000 colony-forming units/mL. Thus, the nuclease-activatable P2&3TT probe distinguishes clinical S. aureus-positive blood cultures from non-S. aureus-positive blood cultures and culture-negative blood, accurately, rapidly and with high sensitivity. We conclude that this probe may enhance the diagnosis of SAB.
Conflict of interest statement
J.O.M. is founder and CEO of Nuclease Probe Technologies Inc., and he holds patents on oligonucleotide-based nuclease-activatable probes for detection of bacterial nucleases. G.M.v.D. is CEO, founder and shareholder of AxelaRx/TRACER group, CSO AxelaRx Biosciences Inc. The other authors have no conflicts of interest to disclose.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

