Methyljasmonate and salicylic acid contribute to the control of Tilletia controversa Kühn, causal agent of wheat dwarf bunt
- PMID: 33154472
- PMCID: PMC7645591
- DOI: 10.1038/s41598-020-76210-2
Methyljasmonate and salicylic acid contribute to the control of Tilletia controversa Kühn, causal agent of wheat dwarf bunt
Abstract
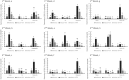
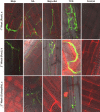
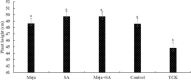
Tilletia controversa Kühn (TCK) is the causal agent of dwarf bunt of wheat, a destructive disease in wheat-growing regions of the world. The role of Meja, SA and Meja + SA were characterized for their control of TCK into roots, coleoptiles and anthers. The response of the defence genes PR-10a, Catalase, COI1-1, COII-2 and HRin1 was upregulated by Meja, SA and Meja + SA treatments, but Meja induced high level of expression compared to SA and Meja + SA at 1, 2, and 3 weeks in roots and coleoptiles, respectively. The severity of TCK effects in roots was greater at 1 week, but it decreased at 2 weeks in all treatments. We also investigated TCK hyphae proliferation into coleoptiles at 3 weeks and into anthers to determine whether hyphae move from the roots to the upper parts of the plants. The results showed that no hyphae were present in the coleoptiles and anthers of Meja-, SA- and Meja + SA-treated plants, while the hyphae were located on epidermal and sub-epidermal cells of anthers. In addition, the severity of hyphae increased with the passage of time as anthers matured. Bunted seeds were observed in the non-treated inoculated plants, while no disease symptoms were observed in the resistance of inducer treatments and control plants. Plant height was reduced after TCK infection compared to that of the treated inoculated and non-inoculated treatments. Together, these results suggested that Meja and SA display a distinct role in activation of defence genes in the roots and coleoptiles and that they eliminate the fungal pathogen movement to upper parts of the plants with the passage of time as the anthers mature.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Wheat Varietal Response to Tilletia controversa J. G. Kühn Using qRT-PCR and Laser Confocal Microscopy.Genes (Basel). 2021 Mar 16;12(3):425. doi: 10.3390/genes12030425. Genes (Basel). 2021. PMID: 33809560 Free PMC article.
-
Characterization of histological changes at the tillering stage (Z21) in resistant and susceptible wheat plants infected by Tilletia controversa Kühn.BMC Plant Biol. 2021 Jan 18;21(1):49. doi: 10.1186/s12870-020-02819-0. BMC Plant Biol. 2021. PMID: 33461490 Free PMC article.
-
Characterization of the wheat cultivars against Tilletia controversa Kühn, causal agent of wheat dwarf bunt.Sci Rep. 2020 Jun 3;10(1):9029. doi: 10.1038/s41598-020-65748-w. Sci Rep. 2020. PMID: 32494028 Free PMC article.
-
Leaf and root glucosinolate profiles of Chinese cabbage (Brassica rapa ssp. pekinensis) as a systemic response to methyl jasmonate and salicylic acid elicitation.J Zhejiang Univ Sci B. 2015 Aug;16(8):696-708. doi: 10.1631/jzus.B1400370. J Zhejiang Univ Sci B. 2015. PMID: 26238545 Free PMC article.
-
Microbiome Signature of Endophytes in Wheat Seed Response to Wheat Dwarf Bunt Caused by Tilletia controversa Kühn.Microbiol Spectr. 2023 Feb 14;11(1):e0039022. doi: 10.1128/spectrum.00390-22. Epub 2023 Jan 10. Microbiol Spectr. 2023. PMID: 36625645 Free PMC article.
Cited by
-
Unveiling Chemical Interactions Between Plants and Fungi Using Metabolomics Approaches.Adv Exp Med Biol. 2023;1439:1-20. doi: 10.1007/978-3-031-41741-2_1. Adv Exp Med Biol. 2023. PMID: 37843803
-
Transcriptomics Analysis of Wheat Tassel Response to Tilletia laevis Kühn, Which Causes Common Bunt of Wheat.Front Plant Sci. 2022 Feb 22;13:823907. doi: 10.3389/fpls.2022.823907. eCollection 2022. Front Plant Sci. 2022. PMID: 35273625 Free PMC article.
-
Metabolomic Analysis of Wheat Grains after Tilletia laevis Kühn Infection by Using Ultrahigh-Performance Liquid Chromatography-Q-Exactive Mass Spectrometry.Metabolites. 2022 Aug 28;12(9):805. doi: 10.3390/metabo12090805. Metabolites. 2022. PMID: 36144210 Free PMC article.
-
Transcriptome mapping related genes encoding PR1 protein involved in necrotic symptoms to soybean mosaic virus infection.Mol Breed. 2023 Jan 16;43(2):7. doi: 10.1007/s11032-022-01351-3. eCollection 2023 Feb. Mol Breed. 2023. PMID: 37313127 Free PMC article.
-
Wheat Varietal Response to Tilletia controversa J. G. Kühn Using qRT-PCR and Laser Confocal Microscopy.Genes (Basel). 2021 Mar 16;12(3):425. doi: 10.3390/genes12030425. Genes (Basel). 2021. PMID: 33809560 Free PMC article.
References
-
- Shiferaw B, et al. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013;5:291–317. doi: 10.1007/s12571-013-0263-y. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

