Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum
- PMID: 33154590
- PMCID: PMC8136619
- DOI: 10.1038/s41590-020-00826-9
Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum
Abstract
Clinical manifestations of COVID-19 caused by the new coronavirus SARS-CoV-2 are associated with age1,2. Adults develop respiratory symptoms, which can progress to acute respiratory distress syndrome (ARDS) in the most severe form, while children are largely spared from respiratory illness but can develop a life-threatening multisystem inflammatory syndrome (MIS-C)3-5. Here, we show distinct antibody responses in children and adults after SARS-CoV-2 infection. Adult COVID-19 cohorts had anti-spike (S) IgG, IgM and IgA antibodies, as well as anti-nucleocapsid (N) IgG antibody, while children with and without MIS-C had reduced breadth of anti-SARS-CoV-2-specific antibodies, predominantly generating IgG antibodies specific for the S protein but not the N protein. Moreover, children with and without MIS-C had reduced neutralizing activity as compared to both adult COVID-19 cohorts, indicating a reduced protective serological response. These results suggest a distinct infection course and immune response in children independent of whether they develop MIS-C, with implications for developing age-targeted strategies for testing and protecting the population.
Conflict of interest statement
Competing Interests statement
The authors have no conflicts with regard to this work.
Figures
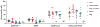



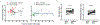
Update of
-
Antibody responses to SARS-CoV2 are distinct in children with MIS-C compared to adults with COVID-19.medRxiv [Preprint]. 2020 Jul 14:2020.07.12.20151068. doi: 10.1101/2020.07.12.20151068. medRxiv. 2020. Update in: Nat Immunol. 2021 Jan;22(1):25-31. doi: 10.1038/s41590-020-00826-9. PMID: 32699861 Free PMC article. Updated. Preprint.
References
-
- Dong Y, et al. Epidemiology of COVID-19 Among Children in China. Pediatrics 145(2020). - PubMed
-
- Wu Z & McGoogan JM Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 323, 1239–1242 (2020). - PubMed
Methods-only References
-
- Ranieri VM, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 307, 2526–2533 (2012). - PubMed
-
- Khemani RG, Smith LS, Zimmerman JJ, Erickson S & Pediatric Acute Lung Injury Consensus Conference, G. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 16, S23–40 (2015). - PubMed
-
- CDC. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). Vol. 2020 Health Alert Network (Health and Human Services, 2020).
Publication types
MeSH terms
Substances
Grants and funding
- R38 CA231577/CA/NCI NIH HHS/United States
- NS105699/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)/International
- U01 AI100119/AI/NIAID NIH HHS/United States
- K08DK122130/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)/International
- K08 DK122130/DK/NIDDK NIH HHS/United States
- AI121349/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
- AI128949/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
- NS091263/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)/International
- AI141686/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
- K23 AI141686/AI/NIAID NIH HHS/United States
- AI146980/U.S. Department of Health & Human Services | National Institutes of Health (NIH)/International
- U19 AI128949/AI/NIAID NIH HHS/United States
- fellowship/Cancer Research Institute (CRI)/International
- AI114736/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
- AI100119/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

