Five-Year Patterns of Diabetic Retinopathy Progression in US Clinical Practice
- PMID: 33154625
- PMCID: PMC7605957
- DOI: 10.2147/OPTH.S275968
Five-Year Patterns of Diabetic Retinopathy Progression in US Clinical Practice
Abstract
Purpose: To characterize the natural course of diabetic retinopathy (DR) in contemporary clinical practice.
Patients and methods: This was a retrospective analysis of US claims data collected between January 1, 2006, and April 30, 2017. Patients aged ≥18 years with continuous medical and prescription insurance coverage for 18 months before DR diagnosis (index date) and for a follow-up period of 5 years were included (N=14,490). The time and risk of progressing to severe nonproliferative DR (NPDR) or proliferative DR (PDR) and of developing diabetic macular edema (DME) were evaluated over 5 years in patients stratified by DR severity at initial diagnosis.
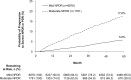
Results: The estimated probability of progressing to severe NPDR or PDR within 5 years of diagnosis was 17.6% for patients with moderate NPDR versus 5.8% for mild NPDR. The probability of developing DME within 5 years was 62.6%, 44.6%, and 28.4% for patients diagnosed with severe NPDR, moderate NPDR, and PDR, respectively, versus 15.6% for mild NPDR. Among those observed to progress, median time to severe NPDR or PDR was approximately 2.0 years in patients with moderate NPDR, whereas median time to DME was approximately 0.5 years in patients with severe NPDR, 1.3 years in moderate NPDR, and 1.6 years in PDR. Relative to mild NPDR, adjusted hazard ratios (95% confidence interval) for progression to severe NPDR or PDR within 5 years were 3.12 (2.61-3.72) in patients with moderate NPDR, and for incident DME were 5.92 (5.13-6.82), 3.54 (3.22-3.91), and 1.96 (1.80-2.14) in patients with severe NPDR, moderate NPDR, and PDR, respectively.
Conclusion: The risk of DR progression and DME over 5 years was highest among patients diagnosed with moderate and severe NPDR, respectively. Our findings reinforce the importance of close monitoring for these patients to avoid unobserved disease progression toward PDR and/or DME.
Keywords: diabetic macular edema; diabetic retinopathy; disease progression; real-world evidence.
© 2020 Moshfeghi et al.
Conflict of interest statement
Andrew Moshfeghi has served as a consultant for Alimera, Allegro, Allergan, Genentech, Inc., Graybug, Novartis, OptiSTENT, Regeneron, Valeant, and Visunex; has served as an investigator for Genentech, Inc., Regeneron, and REGENXBIO Inc.; is a stockholder for OptiSTENT, Pr3vent, and Visunex; has received research funding from Allegro, Genentech, Inc., Novartis, and Regeneron; reports grants and personal fees from Genentech, Inc. during the conduct of the study; reports consulting work and research grants from Genentech, Inc., Novartis, and Regeneron during the conduct of the study; and reports consulting work for and a public equity interest in Ocular Therapeutix during the conduct of the study. Vincent Garmo is an employee of Genentech, Inc.; reports personal fees from and stock in Genentech, Inc. during the conduct of the study; and reports personal fees from and stock in Genentech, Inc. outside the submitted work. Daniel Sheinson and Ibrahim Abbass are employees of Genentech, Inc. Avanti Ghanekar was an employee of Genentech, Inc. during the conduct of the study (current employee of REGENXBIO Inc., Rockville, MD); and reports stock in Genentech, Inc. during the conduct of the study. The authors report no other potential conflicts of interest for this work.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous