Glioblastoma Immune Landscape and the Potential of New Immunotherapies
- PMID: 33154756
- PMCID: PMC7591769
- DOI: 10.3389/fimmu.2020.585616
Glioblastoma Immune Landscape and the Potential of New Immunotherapies
Abstract
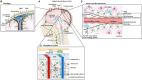
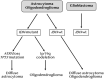
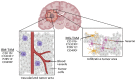
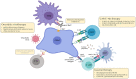
Glioblastoma (GBM) are the most common tumors of the central nervous system and among the deadliest cancers in adults. GBM overall survival has not improved over the last decade despite optimization of therapeutic standard-of-care. While immune checkpoint inhibitors (ICI) have revolutionized cancer care, they unfortunately have little therapeutic success in GBM. Here, we elaborate on normal brain and GBM-associated immune landscapes. We describe the role of microglia and tumor-associated macrophages (TAMs) in immune suppression and highlight the impact of energy metabolism in immune evasion. We also describe the challenges and opportunities of immunotherapies in GBM and discuss new avenues based on harnessing the anti-tumor activity of myeloid cells, vaccines, chimeric antigen receptors (CAR)-T and -NK cells, oncolytic viruses, nanocarriers, and combination therapies.
Keywords: CART-T cell; glioblastoma; immune response; immunotherapy; macrophage.
Copyright © 2020 Daubon, Hemadou, Romero Garmendia and Saleh.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

