Engineering Biomaterials and Approaches for Mechanical Stretching of Cells in Three Dimensions
- PMID: 33154967
- PMCID: PMC7591716
- DOI: 10.3389/fbioe.2020.589590
Engineering Biomaterials and Approaches for Mechanical Stretching of Cells in Three Dimensions
Abstract
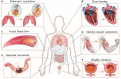
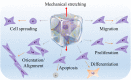
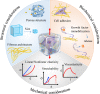
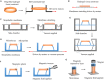
Mechanical stretch is widely experienced by cells of different tissues in the human body and plays critical roles in regulating their behaviors. Numerous studies have been devoted to investigating the responses of cells to mechanical stretch, providing us with fruitful findings. However, these findings have been mostly observed from two-dimensional studies and increasing evidence suggests that cells in three dimensions may behave more closely to their in vivo behaviors. While significant efforts and progresses have been made in the engineering of biomaterials and approaches for mechanical stretching of cells in three dimensions, much work remains to be done. Here, we briefly review the state-of-the-art researches in this area, with focus on discussing biomaterial considerations and stretching approaches. We envision that with the development of advanced biomaterials, actuators and microengineering technologies, more versatile and predictive three-dimensional cell stretching models would be available soon for extensive applications in such fields as mechanobiology, tissue engineering, and drug screening.
Keywords: cell mechanotransduction; hydrogels; mechanobiology; stretch; tissue engineering.
Copyright © 2020 Zhang, Huang and Xu.
Figures
Similar articles
-
Mechanical stretching for tissue engineering: two-dimensional and three-dimensional constructs.Tissue Eng Part B Rev. 2012 Aug;18(4):288-300. doi: 10.1089/ten.TEB.2011.0465. Epub 2012 Mar 28. Tissue Eng Part B Rev. 2012. PMID: 22335794 Free PMC article. Review.
-
Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications.Acta Biomater. 2017 Oct 15;62:42-63. doi: 10.1016/j.actbio.2017.07.028. Epub 2017 Jul 20. Acta Biomater. 2017. PMID: 28736220 Review.
-
A microfabricated platform with hydrogel arrays for 3D mechanical stimulation of cells.Acta Biomater. 2016 Apr 1;34:113-124. doi: 10.1016/j.actbio.2015.11.054. Epub 2015 Nov 29. Acta Biomater. 2016. PMID: 26646540
-
Tissue Regeneration from Mechanical Stretching of Cell-Cell Adhesion.Tissue Eng Part C Methods. 2019 Nov;25(11):631-640. doi: 10.1089/ten.TEC.2019.0098. Epub 2019 Sep 25. Tissue Eng Part C Methods. 2019. PMID: 31407627 Free PMC article. Review.
-
Three-Dimensional Biomaterials with Spatiotemporal Control for Regenerative Tissue Engineering.Acc Chem Res. 2023 Jun 6;56(11):1313-1319. doi: 10.1021/acs.accounts.2c00666. Epub 2023 Apr 27. Acc Chem Res. 2023. PMID: 37103937
Cited by
-
Evaluation of Marine Agarose Biomaterials for Tissue Engineering Applications.Int J Mol Sci. 2021 Feb 15;22(4):1923. doi: 10.3390/ijms22041923. Int J Mol Sci. 2021. PMID: 33672027 Free PMC article.
-
Compound 48/80 increases murine bladder wall compliance independent of mast cells.Sci Rep. 2023 Jan 12;13(1):625. doi: 10.1038/s41598-023-27897-6. Sci Rep. 2023. PMID: 36635439 Free PMC article.
-
Harnessing the potential of hydrogels for advanced therapeutic applications: current achievements and future directions.Signal Transduct Target Ther. 2024 Jul 1;9(1):166. doi: 10.1038/s41392-024-01852-x. Signal Transduct Target Ther. 2024. PMID: 38945949 Free PMC article. Review.
-
Current Advances in 3D Dynamic Cell Culture Systems.Gels. 2022 Dec 16;8(12):829. doi: 10.3390/gels8120829. Gels. 2022. PMID: 36547353 Free PMC article. Review.
-
Dynamic Stimulations with Bioengineered Extracellular Matrix-Mimicking Hydrogels for Mechano Cell Reprogramming and Therapy.Adv Sci (Weinh). 2023 Jul;10(21):e2300670. doi: 10.1002/advs.202300670. Epub 2023 Apr 29. Adv Sci (Weinh). 2023. PMID: 37119518 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

