Cellular Changes in Retinas From Patients With BEST1 Mutations
- PMID: 33154968
- PMCID: PMC7591587
- DOI: 10.3389/fcell.2020.573330
Cellular Changes in Retinas From Patients With BEST1 Mutations
Abstract
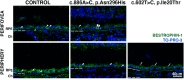
Best disease (BD), also known as vitelliform macular dystrophy, is an inherited disease of the central retina caused by more than 300 pathogenic variants in the BEST1 gene. The phenotype of BD is variable, and there are just a few reports on the histopathology of eyes from donors with BD. Here, we describe the histopathological comparison of donor's eyes from two patients with BD. Eyes obtained from 85-year-old (donor 1) and 65-year-old (donor 2) donors were fixed within 25 h postmortem. Perifoveal and peripheral retinal regions were processed for histology and immunocytochemistry using retinal-specific and retinal pigment epithelium (RPE)-specific antibodies. Three age-matched normal eyes were used as controls. DNA was obtained from donor blood samples. Sequence analysis of the entire BEST1 coding region was performed and identified a c.886A > C (p.Asn296His) variant in donor 1 and a c.602T > C (p.Ile201Thr) variant in donor 2; both mutations were heterozygous. Fundus examination showed that donor 1 displayed a macular lesion with considerable scarring while donor 2 displayed close to normal macular morphology. Our studies of histology and molecular pathology in the perifovea and periphery of these two BD donor eyes revealed panretinal abnormalities in both photoreceptors and RPE cellular levels in the periphery; donor 1 also displayed macular lesion. Our findings confirm the phenotypic variability of BD associated with BEST1 variants.
Keywords: BEST1 gene; best disease; histopathology; photoreceptors; retinal pigment epithelium.
Copyright © 2020 Bonilha, Bell, DeBenedictis, Hagstrom, Fishman and Hollyfield.
Figures




References
-
- Bonilha V. L., Rayborn M. E., Bell B. A., Marino M. J., Pauer G. J., Beight C. D., et al. (2015). Histopathological comparison of eyes from patients with autosomal recessive retinitis pigmentosa caused by novel EYS mutations. Graefes Arch. Clin. Exp. Ophthalmol. 253 295–305. 10.1007/s00417-014-2868-z - DOI - PMC - PubMed
-
- Bonilha V. L., Rodriguez-Boulan E. (2001). Polarity and developmental regulation of two PDZ proteins in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 42 3274–3282. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

