Baseline results of a living systematic review for COVID-19 clinical trial registrations
- PMID: 33154979
- PMCID: PMC7610178
- DOI: 10.12688/wellcomeopenres.15933.1
Baseline results of a living systematic review for COVID-19 clinical trial registrations
Abstract
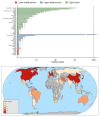
Background: Since the coronavirus disease 2019 (COVID-19) outbreak was first reported in December 2019, many independent trials have been planned that aim to answer similar questions. Tools allowing researchers to review studies already underway can facilitate collaboration, cooperation and harmonisation. The Infectious Diseases Data Observatory (IDDO) has undertaken a living systematic review (LSR) to provide an open, accessible and frequently updated resource summarising characteristics of COVID-19 study registrations. Methods: Review of all eligible trial records identified by systematic searches as of 3 April 2020 and initial synthesis of clinical study characteristics were conducted. In partnership with Exaptive, an open access, cloud-based knowledge graph has been created using the results. Results: There were 728 study registrations which met eligibility criteria and were still active. Median (25 th, 75 th percentile) sample size was 130 (60, 400) for all studies and 134 (70, 300) for RCTs. Eight lower middle and low income countries were represented among the planned recruitment sites. Overall 109 pharmacological interventions or advanced therapy medicinal products covering 23 drug categories were studied. Majority (57%, 62/109) of them were planned only in one study arm, either alone or in combination with other interventions. There were 49 distinct combinations studied with 90% (44/49) of them administered in only one or two study arms. The data and interactive platform are available at https://iddo.cognitive.city/. Conclusions: Baseline review highlighted that the majority of investigations in the first three months of the outbreak were small studies with unique treatment arms, likely to be unpowered to provide solid evidence. The continued work of this LSR will allow a more dependable overview of interventions tested, predict the likely strength of evidence generated, allow fast and informative filtering of relevant trials for specific user groups and provide the rapid guidance needed by investigators and funders to avoid duplication of efforts.
Keywords: COVID-19; Living systematic review; SARS2-CoV2; clinical trials; coronavirus; emerging infections.
Copyright: © 2020 Maguire BJ et al.
Conflict of interest statement
No competing interests were disclosed.
Figures




References
-
- WHO: International Clinical Trials Registry Platform (ICTRP) Data Providers.2020. Reference Source
-
- Philippine Health Research Registry. Reference Source
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

