Prospective Multicenter Study of Circulating Tumor Cell AR-V7 and Taxane Versus Hormonal Treatment Outcomes in Metastatic Castration-Resistant Prostate Cancer
- PMID: 33154984
- PMCID: PMC7608579
- DOI: 10.1200/PO.20.00200
Prospective Multicenter Study of Circulating Tumor Cell AR-V7 and Taxane Versus Hormonal Treatment Outcomes in Metastatic Castration-Resistant Prostate Cancer
Abstract
Purpose: Androgen receptor splice variant 7 (AR-V7) detection in circulating tumor cells (CTCs) is associated with a low probability of response and short progression-free (PFS) and overall survival (OS) in men with metastatic castration-resistant prostate cancer (mCRPC) treated with enzalutamide or abiraterone. However, it is unclear whether such men benefit from taxane chemotherapy.
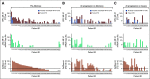
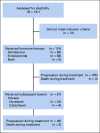
Patients and methods: PROPHECY is a multicenter prospective blinded study of patients with poor-risk mCRPC starting abiraterone or enzalutamide and observed through subsequent progression and taxane chemotherapy. We assessed AR-V7 status using the Johns Hopkins modified AdnaTest CTC AR-V7 messenger RNA assay and the Epic Sciences CTC nuclear-localized AR-V7 protein assay before treatment. The primary objective was to validate the independent prognostic value of CTC AR-V7 status based on radiographic/clinical PFS. OS, confirmed prostate-specific antigen (PSA), and objective radiologic responses were secondary end points.
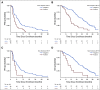
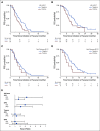
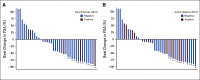
Results: We enrolled 118 men with mCRPC treated with abiraterone or enzalutamide, 51 of whom received subsequent docetaxel or cabazitaxel. Pretreatment CTC AR-V7 status by the Johns Hopkins and Epic Sciences assays was independently associated with worse PFS (hazard ratio [HR], 1.7; 95% CI, 1.0 to 2.9 and HR, 2.1; 95% CI, 1.0 to 4.4, respectively) and OS (HR, 3.3; 95% CI, 1.7 to 6.3 and HR, 3.0; 95% CI, 1.4 to 6.3, respectively) and a low probability of confirmed PSA responses, ranging from 0% to 11%, during treatment with abiraterone or enzalutamide. At progression, subsequent CTC AR-V7 detection was not associated with an inferior PSA or radiographic response or worse PFS or OS with subsequent taxane chemotherapy after adjusting for CellSearch CTC enumeration and clinical prognostic factors.
Conclusion: Detection of AR-V7 in CTCs by two different blood-based assays is independently associated with shorter PFS and OS with abiraterone or enzalutamide, but such men with AR-V7-positive disease still experience clinical benefits from taxane chemotherapy.
© 2020 by American Society of Clinical Oncology.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center. Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments). Andrew J. ArmstrongHonoraria: Dendreon, Janssen Oncology Consulting or Advisory Role: Bayer, Sanofi, Dendreon, Medivation, Janssen Biotech, Pfizer, Astellas Scientific and Medical Affairs, Clovis Oncology, AstraZeneca Speakers’ Bureau: Dendreon, Bayer Research Funding: Dendreon (Inst), Sanofi (Inst), Bayer (Inst), Pfizer (Inst), Novartis (Inst), Janssen Oncology (Inst), Medivation (Inst), Astellas Pharma (Inst), Gilead Sciences (Inst), Roche/Genentech (Inst), Active Biotech (Inst), Bristol Myers Squibb (Inst), Constellation Pharmaceuticals (Inst), Merck (Inst) Patents, Royalties, Other Intellectual Property: Circulating tumor cell novel capture technology (Inst) Travel, Accommodations, Expenses: Dendreon, Janssen Biotech, Bayer, Astellas Scientific and Medical AffairsJun LuoConsulting or Advisory Role: Sun Pharma, Janssen Oncology, Tolero Pharmaceuticals Research Funding: Sanofi (Inst), Orion Pharma (Inst), Mirati Therapeutics (Inst), Gilead Sciences (Inst), Astellas Pharma (Inst), Constellation Pharmaceuticals (Inst), Calibr (Inst), Cardiff Oncology (Inst) Patents, Royalties, Other Intellectual Property: Coinventor of a technology assigned to Johns Hopkins University, which licensed to Tokai Pharmaceuticals (Inst); coinventor of a technology licensed to Qiagen (Inst); coinventor of a technology licensed to A&G Pharmaceuticals (Inst)David M. NanusConsulting or Advisory Role: Roche/Genentech Research Funding: Novartis (Inst), Boehringer Ingelheim (Inst), Zenith Epigenetics (Inst), AstraZeneca (Inst), Immumedics (Inst), Janssen (Inst), Clovis Oncology (Inst), Pfizer (Inst)Paraskevi GiannakakouEmployment: Novartis (I) Stock and Other Ownership Interests: Novartis (I) Patents, Royalties, Other Intellectual Property: Coinventor on international patent application docket No. 1676.083WO1“Identifying taxane sensitivity in prostate cancer patients”Russell Z. SzmulewitzHonoraria: Astellas Pharma Consulting or Advisory Role: AstraZeneca, AbbVie, Exelixis, Merck, Amgen, Janssen Oncology, Sanofi, Astellas Pharma, Pfizer Research Funding: AbbVie, Astellas Pharma, Incyte, Macrogenics, Janssen Oncology Patents, Royalties, Other Intellectual Property: Coinventor on patent licensed by University of Chicago to Corcept Therapeutics for combination AR/GR inhibition in prostate cancer Travel, Accommodations, Expenses: Corcept TherapeuticsDaniel C. DanilaHonoraria: Angle, Bayer, ScreenCell, Janssen Oncology, Pfizer, AxImmune, Pfizer, Clovis Oncology, Astellas Pharma Consulting or Advisory Role: Angle, Bayer, Sanador, AxImmune, Pfizer, Clovis Oncology, Astellas Pharma, Janssen Scientific Affairs Research Funding: Prostate Cancer Foundation, Genentech, Janssen Research & Development (Inst) Patents, Royalties, Other Intellectual Property: Gene expression profile associated with prostate cancer Travel, Accommodations, Expenses: Cambridge Healthtech Institute, Prostate Cancer Foundation, ScreenCell, StopCancer, American Austrian Open Medical Institute, Janssen Biotech, Genzyme, Pfizer, Janssen Scientific Affairs, Astellas PharmaPatrick HealyConsulting or Advisory Role: ParexelWilliam R. BerryHonoraria: Pfizer, Merck, Genomic Health, Janssen Oncology Consulting or Advisory Role: Merck, Pfizer, Genomic Health, Janssen Oncology Research Funding: Merck (Inst) Travel, Accommodations, Expenses: Merck, Pfizer, Genomic Health, Janssen OncologyTian ZhangLeadership: Capio BioSciences (I), Archimmune Therapeutics (I) Stock and Other Ownership Interests: Capio Biosciences (I), Archimmune Therapeutics (I), Nanorobotics (I) Honoraria: Exelixis, Genentech/Roche, MJH Life Sciences, Pacific Genuity Consulting or Advisory Role: Janssen, Genentech/Roche, Sanofi, Exelixis, AstraZeneca, Pfizer, Bristol Myers Squibb, Foundation Medicine, Pharmacyclics, Amgen, Merck, Seattle Genetics Speakers’ Bureau: Exelixis, Genentech/Roche, Genomic Health, Sanofi Research Funding: Janssen (Inst), Acerta Pharma (Inst), Pfizer (Inst), Merrimack (Inst), Stem CentRx (Inst), Novartis (Inst), OmniSeq (Inst), Personal Genome Diagnostics (Inst), Regeneron (Inst), Merck (Inst), Mirati Therapeutics (Inst), Astellas Pharma Patents, Royalties, Other Intellectual Property: Circulating tumor cell novel capture by c-MET technology (Inst); prochelators as targeted prodrugs for prostate cancer (Inst) Travel, Accommodations, Expenses: Acerta Pharma, Genomic Health, AstraZenecaMichael R. HarrisonConsulting or Advisory Role: Bayer, Sanofi, Exelixis, Genentech, Argos Therapeutics, Fujifilm, Janssen Oncology, AstraZeneca, Pfizer, Bristol Myers Squibb Speakers’ Bureau: Genentech, Exelixis Research Funding: Argos Therapeutics (Inst), Bristol Myers Squibb (Inst), Genentech (Inst), Pfizer (Inst), Medivation/Astellas Pharma (Inst), Merck (Inst), Clovis Oncology (Inst), Acerta Pharma (Inst), AstraZeneca (Inst)Changxue LuPatents, Royalties, Other Intellectual Property: Inventor of the patent that was licensed to Qiagen and received royaltyJoseph D. SchonhoftEmployment: Epic Sciences Stock and Other Ownership Interests: Epic SciencesHoward I. ScherLeadership: Asterias Biotherapeutics Stock and Other Ownership Interests: Asterias Biotherapeutics Honoraria: Research to Practice Consulting or Advisory Role: Janssen Biotech, Amgen, Janssen Research & Development, Menarini Silicon Biosystems, WIRB-Copernicus Group, ESSA, Sanofi, Ambry Genetics, Konica Minolta, Pfizer, Bayer Research Funding: Janssen (Inst), Illumina (Inst), Epic Sciences (Inst), Menarini Silicon Biosystems (Inst), Thermofisher Scientific Biomarkers (Inst) Travel, Accommodations, Expenses: Asterias Biotherapeutics, Menarini Silicon Biosystems, Amgen, WIRB-Copernicus Group, Konica Minolta, ESSA, Prostate Cancer Foundation, Sanofi, Bayer, Phosplatin TherapeuticsRichard WenstrupEmployment: Epic Sciences Leadership: Epic Sciences Stock and Other Ownership Interests: Epic Sciences Consulting or Advisory Role: Blueprint Genetics, Resolys Patents, Royalties, Other Intellectual Property: Patent royalties from AssurexHealth Travel, Accommodations, Expenses: Epic SystemsScott T. TagawaConsulting or Advisory Role: Medivation, Astellas Pharma, Dendreon, Janssen, Bayer, Genentech, Endocyte, Immunomedics, Karyopharm Therapeutics, AbbVie, Tolmar, QED, Amgen, Sanofi, Pfizer, Clovis Oncology, Novartis, Genomic Health, POINT Biopharma Research Funding: Lilly (Inst), Sanofi (Inst), Janssen (Inst), Astellas Pharma (Inst), Progenics (Inst), Millennium Pharmaceuticals (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), Dendreon (Inst), Rexahn Pharmaceuticals (Inst), Bayer (Inst), Genentech (Inst), Newlink Genetics (Inst), Inovio Pharmaceuticals (Inst), AstraZeneca (Inst), Immunomedics (Inst), Novartis (Inst), AVEO (Inst), Boehringer Ingelheim (Inst), Merck (Inst), Stem CentRx (Inst), Karyopharm Therapeutics (Inst), AbbVie (Inst), Medivation (Inst), Endocyte (Inst), Exelixis (Inst), Clovis Oncology (Inst) Travel, Accommodations, Expenses: Sanofi, Immunomedics, Amgen Uncompensated Relationships: Telix Pharmaceuticals, ATLAB Pharma, Phosplatin TherapeuticsEmmanuel S. AntonarakisHonoraria: Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, Astellas Pharma, Merck, AstraZeneca, Clovis Oncology Consulting or Advisory Role: Sanofi, Dendreon, Janssen Biotech, ESSA, Merck, AstraZeneca, Clovis Oncology, Lilly, Bayer Research Funding: Janssen Biotech (Inst), Johnson & Johnson (Inst), Sanofi (Inst), Dendreon (Inst), Aragon Pharmaceuticals (Inst), Exelixis (Inst), Millennium Pharmaceuticals (Inst), Genentech (Inst), Novartis (Inst), Astellas Pharma (Inst), Tokai Pharmaceuticals (Inst), Merck (Inst), AstraZeneca (Inst), Clovis Oncology (Inst), Constellation Pharmaceuticals (Inst) Patents, Royalties, Other Intellectual Property: Coinventor of a biomarker technology that has been licensed to Qiagen Travel, Accommodations, Expenses: Sanofi, Dendreon, MedivationDaniel J. GeorgeLeadership: Capio BioSciences Honoraria: Sanofi, Bayer, Exelixis, EMD Serono, OncLive, Pfizer, UroToday, Acceleron Pharma, American Association for Cancer Research, Axess Oncology, Janssen Oncology, Millennium Medical Publishing Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Sanofi, Astellas Pharma, Innocrin Pharma, Bristol Myers Squibb, Genentech, Janssen, Merck Sharp & Dohme, Myovant Sciences, AstraZeneca, Michael J. Hennessy Associates, Vizuri Health Sciences Speakers’ Bureau: Sanofi, Bayer, Exelixis Research Funding: Exelixis (Inst), Janssen Oncology (Inst), Novartis (Inst), Pfizer (Inst), Astellas Pharma (Inst), Bristol Myers Squibb (Inst), Acerta Pharma (Inst), Bayer (Inst), Dendreon (Inst), Innocrin Pharma (Inst), Calithera Biosciences (Inst), Sanofi (Inst) Travel, Accommodations, Expenses: Bayer, Exelixis, Merck, Pfizer, Sanofi, Janssen Oncology, UroTodaySusan HalabiEmployment: ASCO Targeted Agent and Profiling Utilization Registry Consulting or Advisory Role: Eisai, Ferring Pharmaceuticals, Bayer No other potential conflicts of interest were reported.
Figures
References
-
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376:1147–1154. - PubMed
-
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. - PubMed
-
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381:2506–2518. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

