Ketamine: A tale of two enantiomers
- PMID: 33155503
- PMCID: PMC7859674
- DOI: 10.1177/0269881120959644
Ketamine: A tale of two enantiomers
Abstract
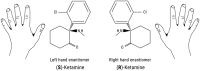
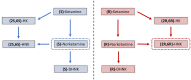
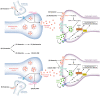
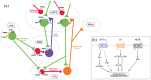
The discovery of the rapid antidepressant effects of the dissociative anaesthetic ketamine, an uncompetitive N-Methyl-D-Aspartate receptor antagonist, is arguably the most important breakthrough in depression research in the last 50 years. Ketamine remains an off-label treatment for treatment-resistant depression with factors that limit widespread use including its dissociative effects and abuse potential. Ketamine is a racemic mixture, composed of equal amounts of (S)-ketamine and (R)-ketamine. An (S)-ketamine nasal spray has been developed and approved for use in treatment-resistant depression in the United States and Europe; however, some concerns regarding efficacy and side effects remain. Although (R)-ketamine is a less potent N-Methyl-D-Aspartate receptor antagonist than (S)-ketamine, increasing preclinical evidence suggests (R)-ketamine may have more potent and longer lasting antidepressant effects than (S)-ketamine, alongside fewer side effects. Furthermore, a recent pilot trial of (R)-ketamine has demonstrated rapid-acting and sustained antidepressant effects in individuals with treatment-resistant depression. Research is ongoing to determine the specific cellular and molecular mechanisms underlying the antidepressant actions of ketamine and its component enantiomers in an effort to develop future rapid-acting antidepressants that lack undesirable effects. Here, we briefly review findings regarding the antidepressant effects of ketamine and its enantiomers before considering underlying mechanisms including N-Methyl-D-Aspartate receptor antagonism, γ-aminobutyric acid-ergic interneuron inhibition, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic receptor activation, brain-derived neurotrophic factor and tropomyosin kinase B signalling, mammalian target of rapamycin complex 1 and extracellular signal-regulated kinase signalling, inhibition of glycogen synthase kinase-3 and inhibition of lateral habenula bursting, alongside potential roles of the monoaminergic and opioid receptor systems.
Keywords: (R)-ketamine; (S)-ketamine; 5-HT; AMPA receptor; BDNF; ERK; GSK-3; Ketamine; NMDA receptor; TrkB; depression; dopamine; mTORC1; opioid receptor.
Conflict of interest statement
Figures

References
-
- Aalto S, Hirvonen J, Kajander J, et al. (2002) Ketamine does not decrease striatal dopamine D2 receptor binding in man. Psychopharmacology (Berl) 164: 401–406. - PubMed
-
- Aalto S, Ihalainen J, Hirvonen J, et al. (2005) Cortical glutamate-dopamine interaction and ketamine-induced psychotic symptoms in man. Psychopharmacology (Berl) 182: 375–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

